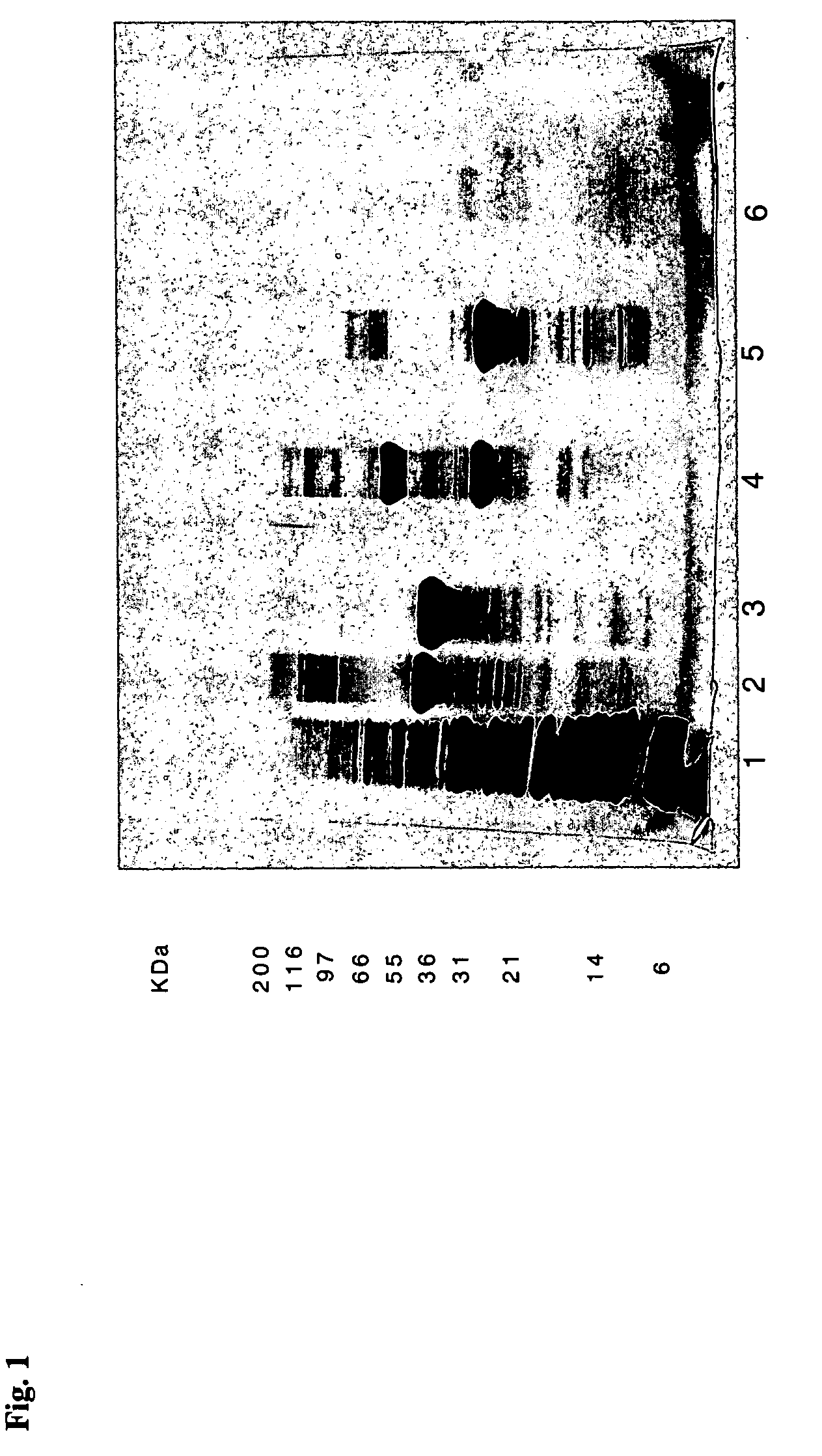
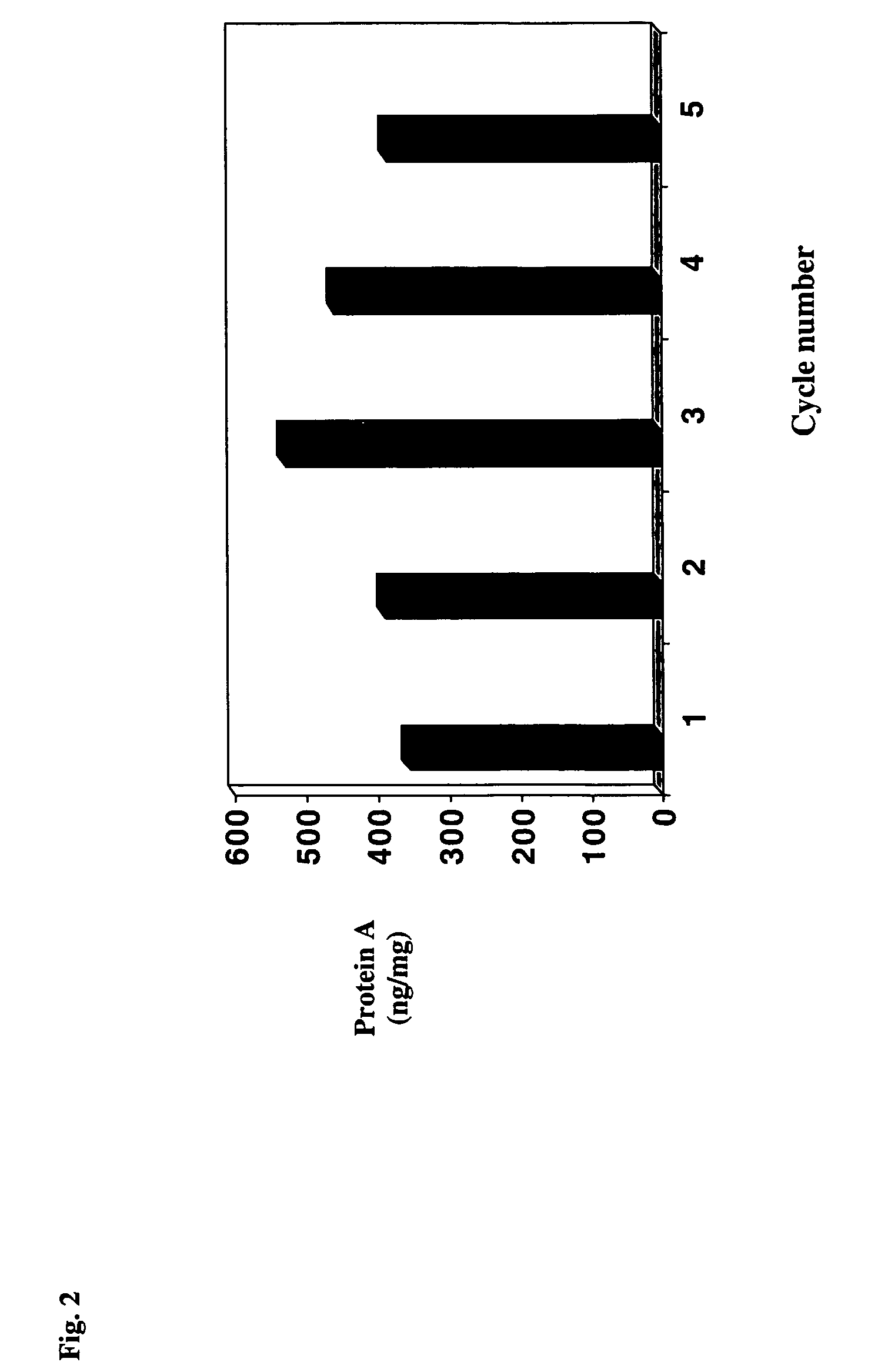
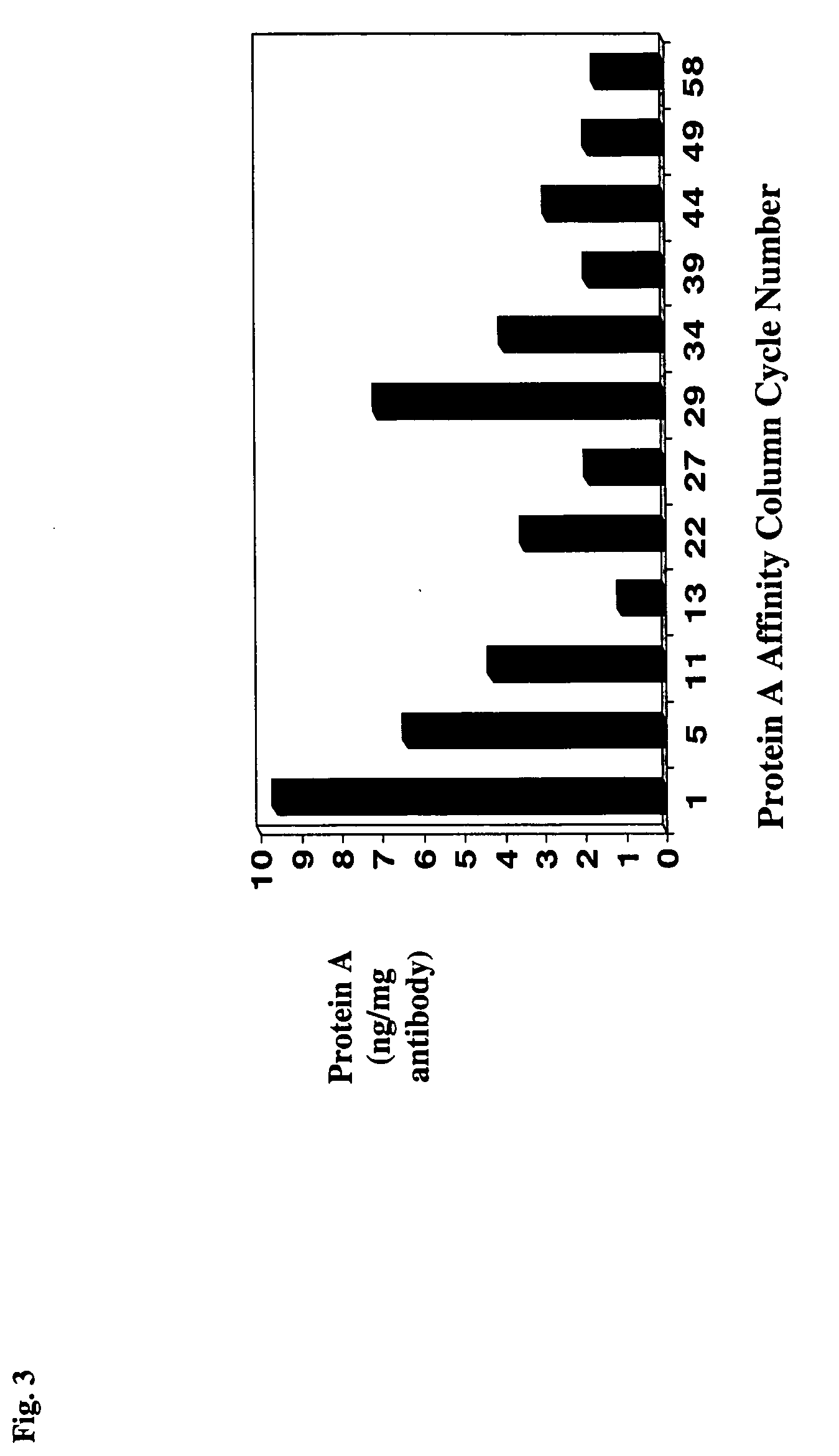
Protein a chromatography
a chromatography and protein technology, applied in the field of protein a chromatography, can solve the problems of increasing the leakage rate of recombinant protein a matrices, difficult to remove, and the inevitably subject of protein a affinity columns to some degree of ligand leakage from the column, so as to achieve the effect of increasing the ionic strength and increasing the throughpu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] It is an object of the present invention to devise another method for separating protein A or protein A fragments from antibody, preferably an IgG, which method avoids the disadvantages of the prior art. According to the present invention, such object is solved according to the independent claims 1 and 9.
[0013] According to the present invention, a method of purifying an antibody is devised which method comprises the steps of: firstly, purifying an antibody by means of protein A affinity chromatography wherein the protein A is a native protein A or a functional derivative thereof.
[0014] Secondly, loading the purified antibody on an ion exchange material under conditions which allow for binding of the protein A or its functional derivative and thirdly, collecting the antibody, preferably collecting at least 70%, more preferably collecting at least 80%, most preferably collecting at least 90% of the amount of antibody loaded onto the ion exchange material in the flow-through ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com