Using heat shock proteins to improve the therapeutic benefit of a non-vaccine treatment modality
a non-vaccine treatment and protein technology, applied in the field of using heat shock proteins, can solve the problems of inconsistent symptoms and abnormal physical findings, difficult to achieve long-term efficacy and toxicity, and still complicated practice, and achieve the effect of improving the therapeutic benefit of non-vaccine and improving the therapeutic profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
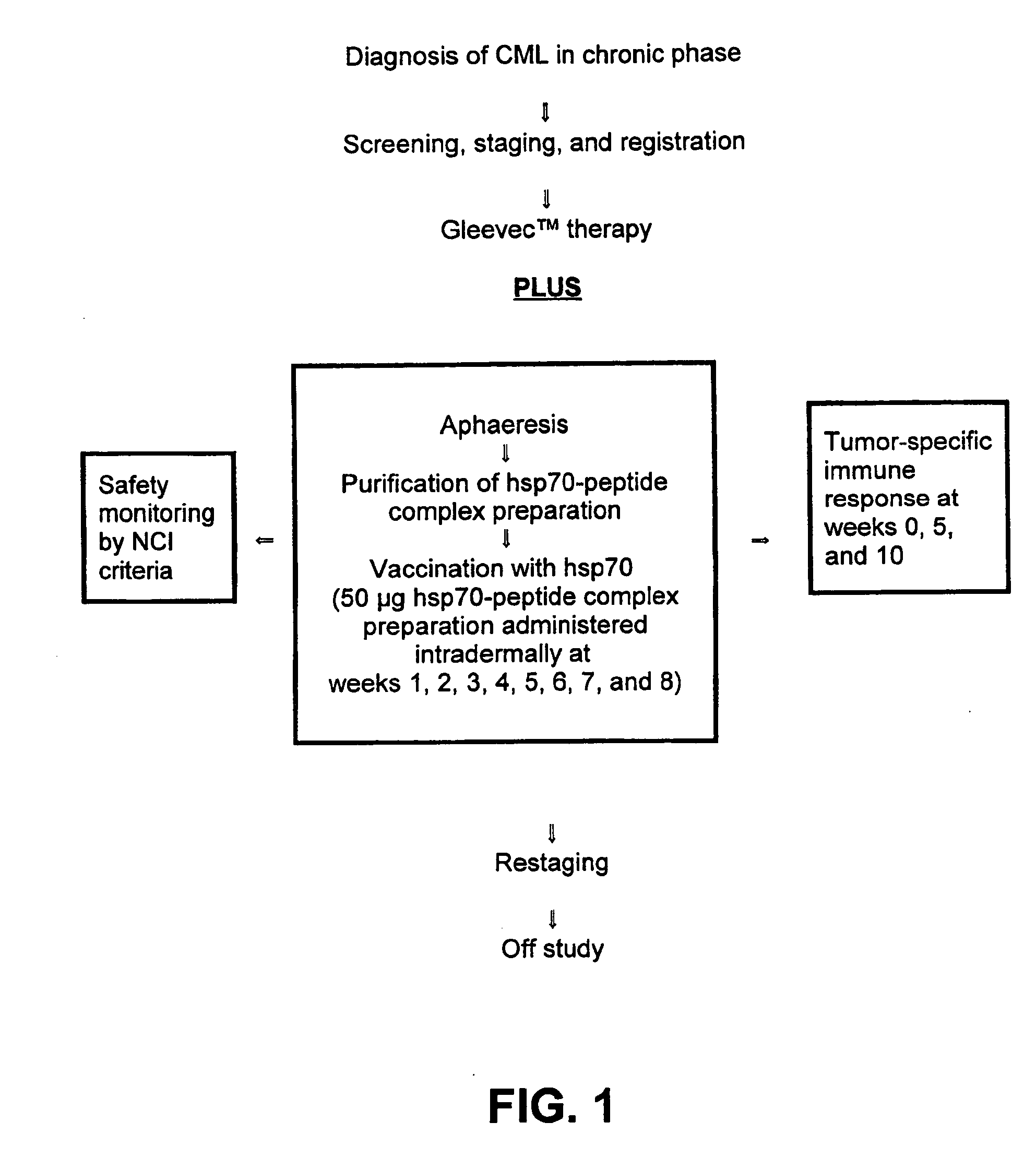
[0051] The present invention is based, in part, on the recognition that an HSP preparation can enhance or improve the therapeutic benefit of non-vaccine treatment modalities or therapeutic modalities for treatment of cancer or infectious diseases. Thus, the present invention encompasses methods and compositions that comprise administering an HSP preparation in combination with a non-vaccine treatment modality. Also encompassed are methods and compositions that comprise administering an α2M preparation in combination with a non-vaccine treatment modality. In particular, the invention encompasses methods of treatment and compositions that provide a better therapeutic profile than that of an HSP preparation or α2M preparation administered alone or a non-vaccine treatment modality administered alone. The source of the HSP or α2M is preferably an eukaryote, and most preferably a mammal. The subject receiving the treatment is preferably a mammal including, but not limited to, domestic ani...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com