Oncoprotein protein kinase
a technology of oncoprotein and kinase, which is applied in the direction of transferases, peptide/protein ingredients, instruments, etc., can solve the problems of overexpression of a particular gene, affecting healthy cells as well as neoplastic cells, and prone to act indiscriminately,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Plasmids and Expression of GST Fusion Proteins
[0097] The glutathione-S-transferase (GST)-cJun expression vector, pGEX2T-cJun(wt), was constructed by inserting a filled-in BspHI-PstI fragment (encoding AA 1-223) from RSV-cJun(BspHI) into the SmaI site of pGEX2T (Pharmacia). RSV-cJun(BspHI) was constructed by changing the translation initiation sequence CTATGA of RSV-cJun to TCATGA by site-directed mutagenesis. The GSTcJun(Ala63 / 67)(BspHI) expression vector was derived in the same manner from RSV-cJun(Ala63 / 73) (Smeal, et al., supra, 1991) and was used to construct pGEX2T-cJun(Ala 63 / 67). The various GSTcJun truncation mutants were constructed using the polymerase chain reaction (PCR) to amplify various portions of c-Jun coding region. The sequences of the primers are indicated below:
N-terminal primers:(SEQ ID NO: 2)TCTGCAGGATCCCCATGACTGCAAAGATGGAAACG (underlinedcodon: amino acid 1);(SEQ ID NO: 3)TCTGCAGGATCCCCGACGATGCCCTCAACGCCTC (a.a.11);(SEQ ID NO: 4)TCTGCAGGATCCCCGAGAGCGGACCTTA...
example 2
Kinase Assays
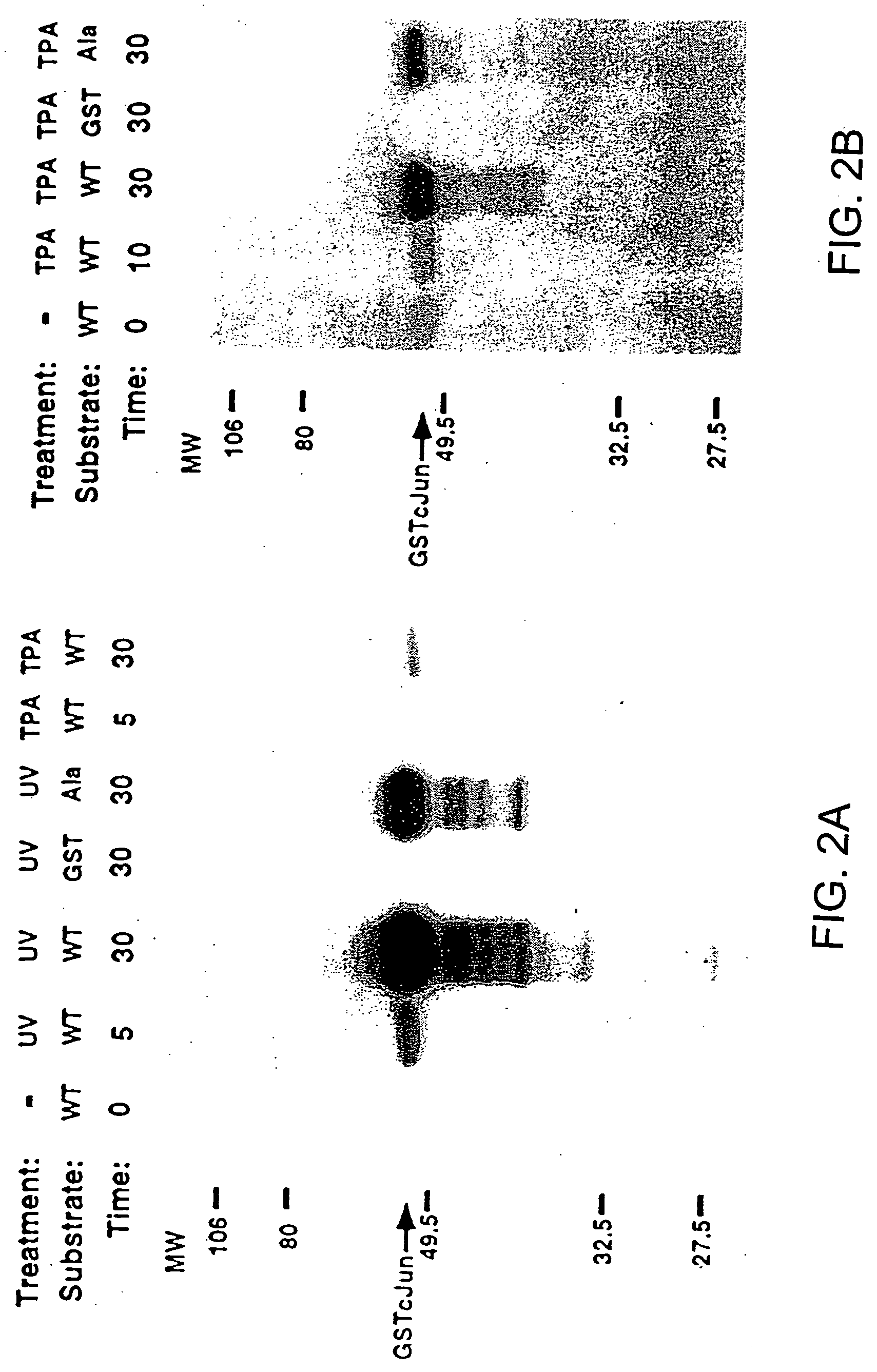
[0104] Cell extracts were diluted so that the final composition of the WCE buffer was 20 mM HEPES pH 7.7, 75 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100, 0.5 mM DTT, 20 mM β-glycerolphosphate, 0.1 mM Na3VO4, 2 μg / ml leupeptin, 100 μg / ml PMSF. The extracts were mixed with 10 μl of GSH-agarose suspension (Sigma) to which 10 μg of either GST or GST-Jun fusion proteins were bound. The mixture was rotated at 4° C. for 3 hours in a microfuge tube and pelleted by centrifugation at 10,000×g for 20 sec. After 4×1 ml washes in HEPES binding buffer (20 mM HEPES pH 7.7, 50 mM NaCl. 2.5 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100), the pelleted beads were resuspended in 30 μl of kinase buffer (20 mM HEPES pH 7.6, 20 mM MgCl2, 20 mM β-glycerolphosphate, 20 μM p-nitrophenyl phosphate, 0.1 mM Na3VO4, 2 mM DTT) containing 20 μM ATP and 5 μCl γ-32P-ATP. After 20 minutes at 30° C. the reaction was terminated by washing with HEPES binding buffer. Phospho...
example 3
Binding of a Protein Kinase to GST-cJun-GSH-Agarose Beads
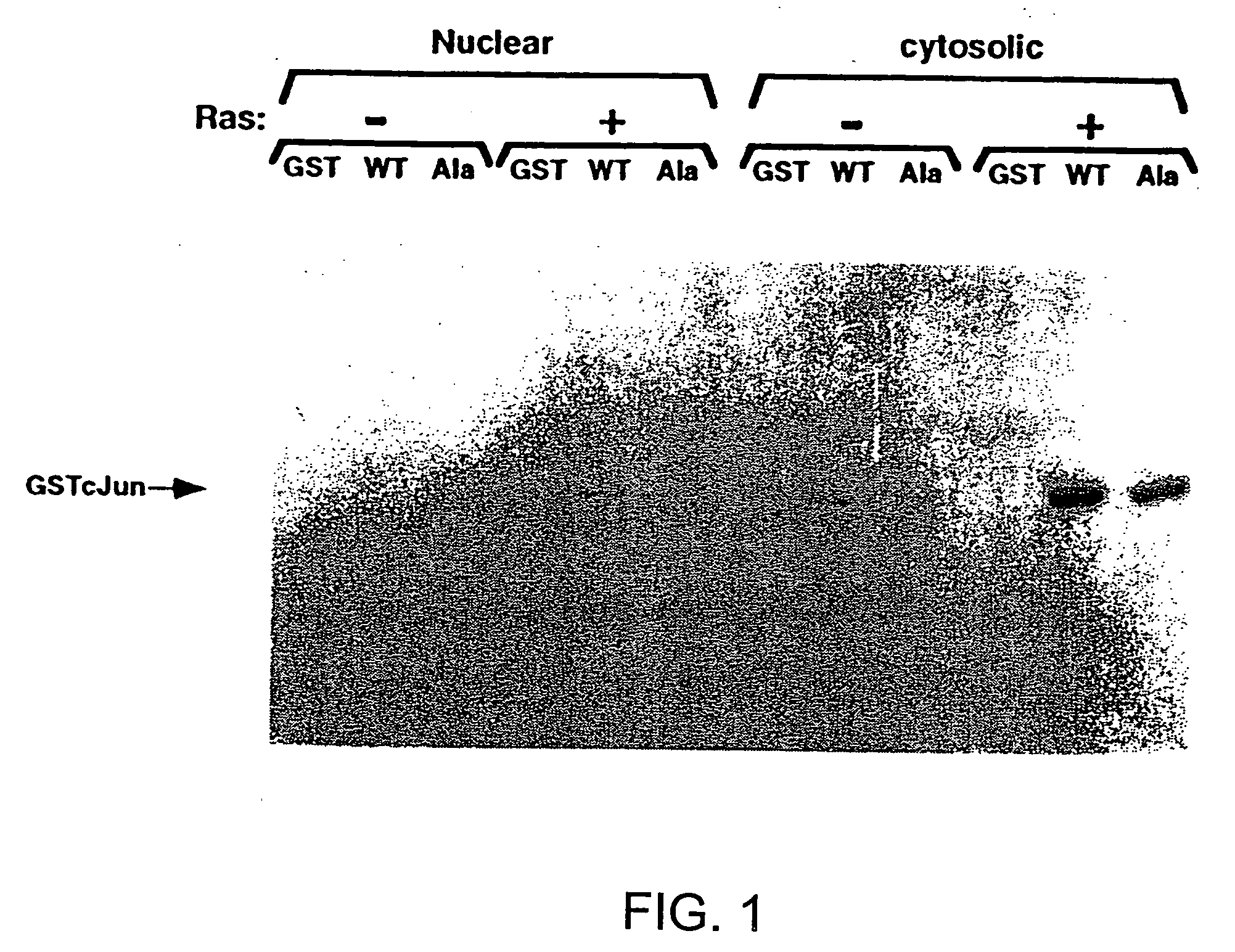
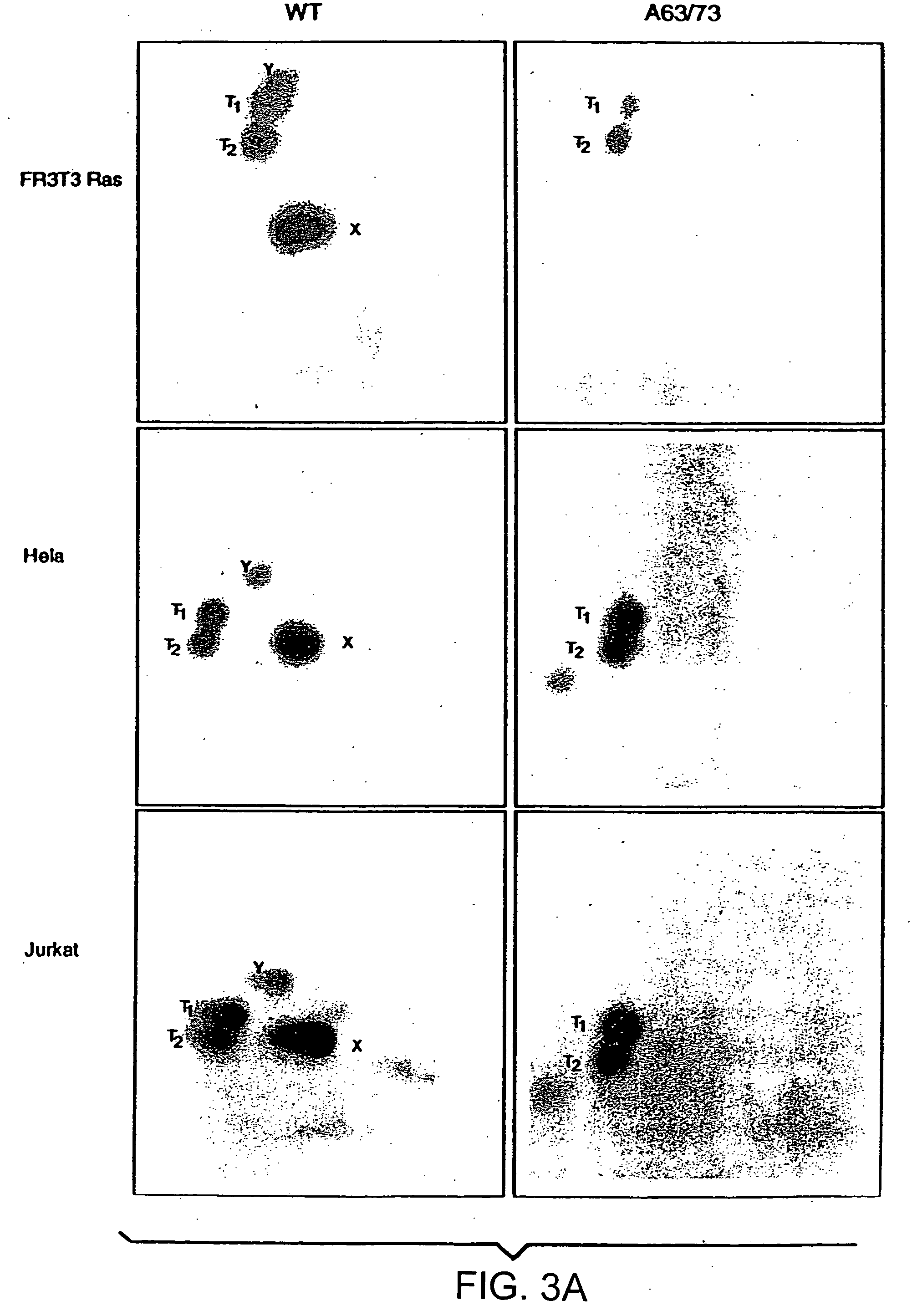
[0106] The fusion protein, GSTcJun(wt), can bind through its GST moiety to glutathione (GSH)-agarose beads to generate an affinity matrix for identification of c-Jun binding proteins, which may include protein kinases. Ha-ras transformation of FR3T3 cells results in increased phosphorylation of c-Jun on Ser 63 and 73 (Binetruy, et al., supra, 1991; Smeal. et al., supra, 1991). Preliminary experiments indicated that transformed cells contained higher levels of c-Jun N-terminal kinase activity, while the levels of c-Jun C-terminal kinase activity remaned unchanged. To develop a more convenient assay for characterizing the c-Jun N-terminal kinase activity, nuclear and cytoplasmic extracts of untransformed and transformed FR3T3 cells were mixed with GSTcJun(wt)-GSH-agarose beads. FRT3T3(−) and Ha-ras-transformed FR3T3(+) cells were kept in 0.5% FCS for 24 hours and harvested to prepare nuclear and cytosolic extracts. These extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com