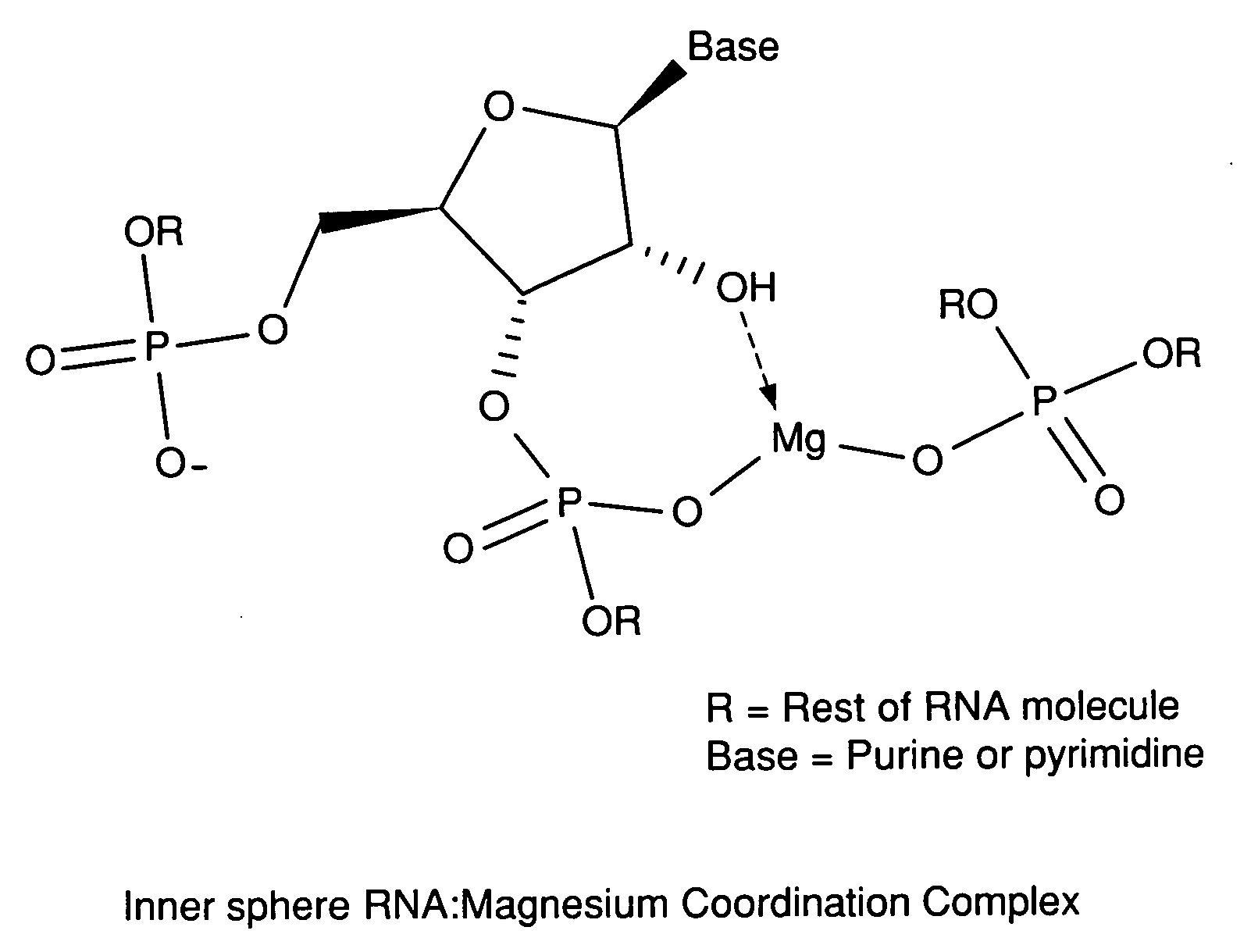
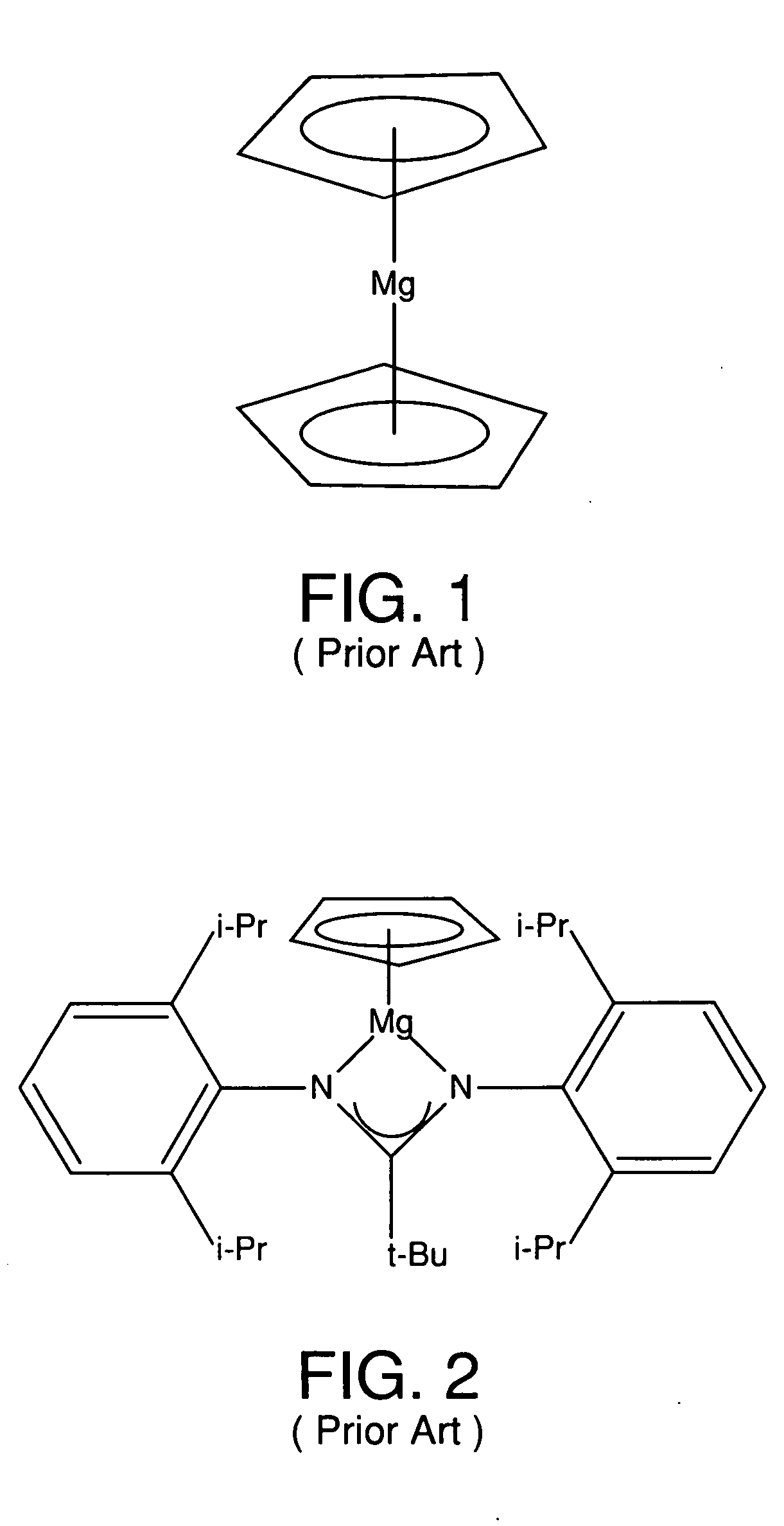
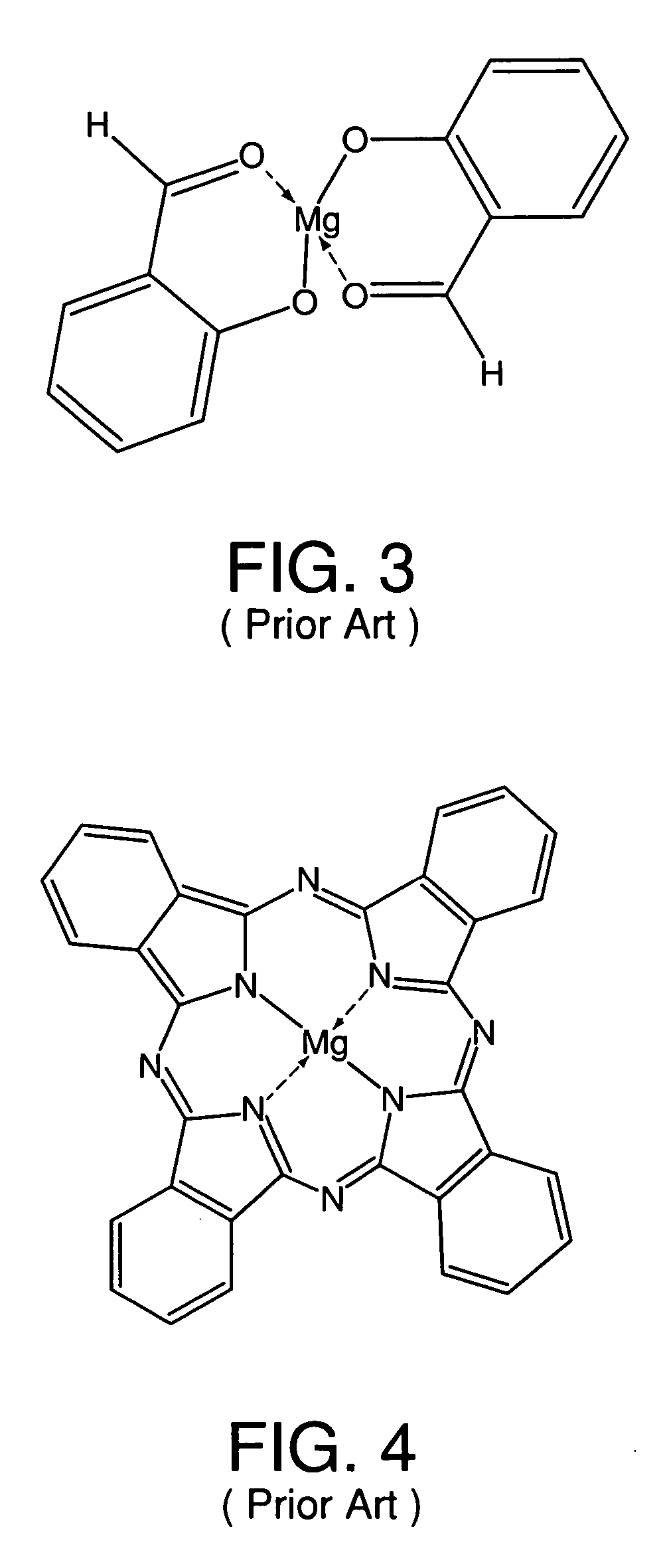
Metal coordinated compositions
a technology of metal coordination and composition, applied in the field of metal coordinated complexes, can solve problems such as difficulties in actual practice, and achieve the effect of further ligation flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
FTIR Analysis of DMSO-Magnesium Complex
[0188] In order to determine which atom of DMSO binds to magnesium and FTIR spectrum was collected of a DMSO-magnesium complex. The FTIR spectrum showed an extra stretch at 954 cm−1, which is indicative of an S═O—Mg stretch. FTIR of T3 complexes were examined for the presence of S═O—Mg, C═O and N—H stretches.
Preparation of Bis(triiodothyroninato)-bis(dimethylsulfoxide)magnesium.
[0189] Triiodothyronine or T3 (218 mg) was dissolved in 4 mL of anhydrous DMSO, after which 0.34 mL of 1 M potassium t-butoxide in t-butanol was added and the solution stirred for 10 minutes. Magnesium chloride (16 mg) was added and the solution stirred overnight. The solution was poured into 10 mL of deionized water to precipitate the product, which was suction filtered and air dried. After an overnight drying under high vacuum the yield was 164 mg of a light beige powder. The product structure was characterized by 1H NMR, FAB-MS and ICP. 1H NMR (DMSO): δ 7.83 (s),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bis | aaaaa | aaaaa |
| bis( | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com