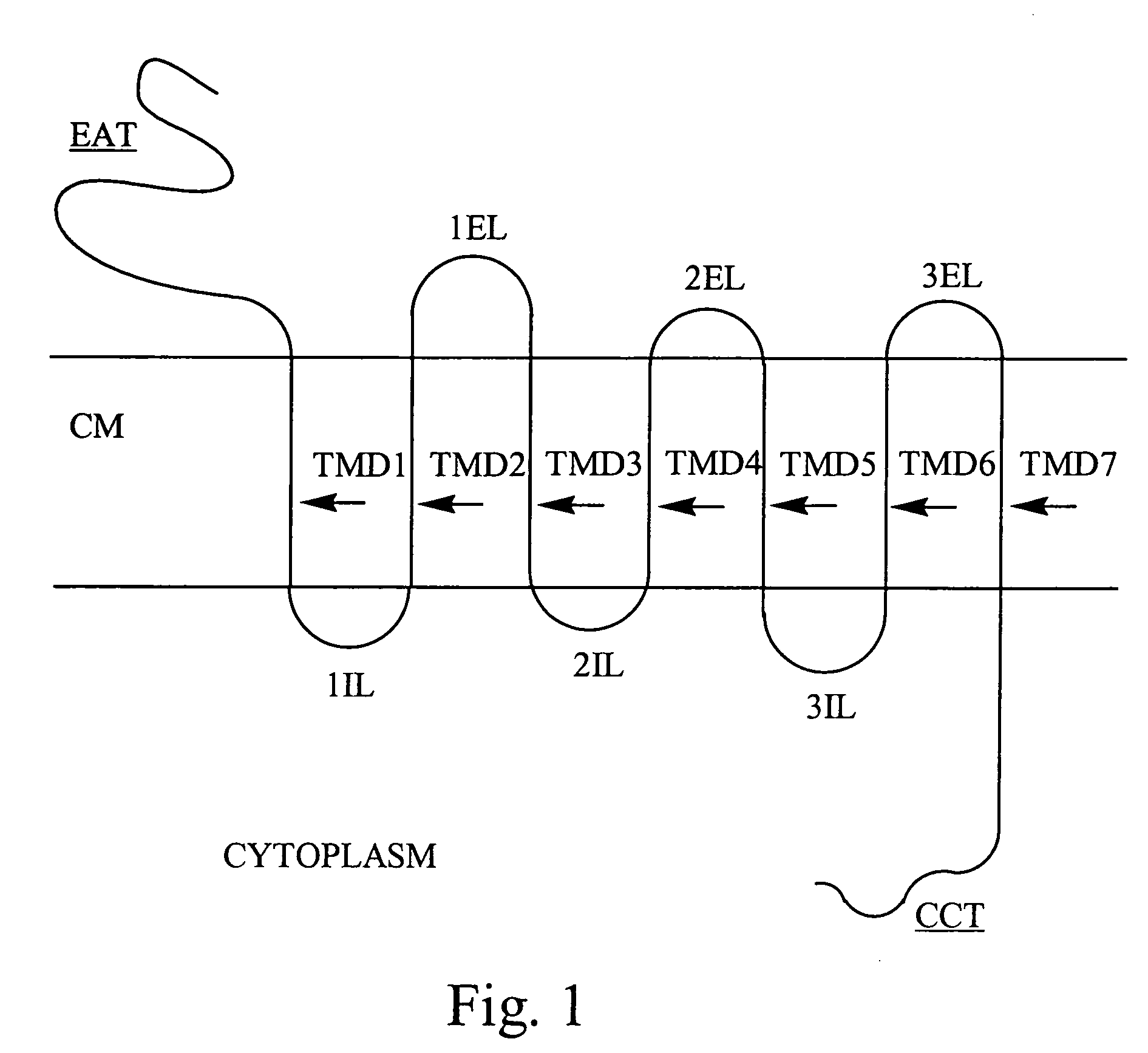
Bivalent binding molecules of 7 transmembrane G protein-coupled receptors
a transmembrane g protein and receptor technology, applied in the field of bivalent binding molecules, can solve the problems of inefficient and/or unpredictable expression levels, difficult purification of receptors, and inability to bind molecules of 7 transmembrane g protein-coupled receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of a Bivalent Binding Molecule to NK1R
[0115] The biological actions of substance P, a neurokinin, is mediated by a neurokinin receptor known as NK1R. This receptor is a member of the 7TM G protein-coupled receptor superfamily. Studies have shown that the extracellular domains of the NK1R comprise the ligand binding sites (Fong, T. M. et al. Journal of Biological Chem. 267:25664-25667 (1992)). This example describes the identification of a bivalent molecule to NK1R having affinity for epitopes in extracellular loop 1 and extracellular loop 2.
a) Synthesis of Peptide Target 1 (Epitope 1).
[0116] Extracellular loop 1 (ECL1) of NK1R is comprised of the 13 amino acid sequence: N-His-Asn-Glu-Trp-Tyr-Tyr-Gly-Leu-Phe-Tyr-Cys-Lys-Phe-C (Fong, et al.) (SEQ. ID NO. 1). ECL1 is synthesized by standard peptide synthesis methods known in the art with an additional Cys at the carboxy terminus (ECL1-Cys) to facilitate coupling to a support. ECL1-Cys is covalently coupled (via a di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com