Metered dose delivery device for liquid and powder agents
a technology of liquid and powder agents and delivery devices, which is applied in the direction of medical inhalators, other medical devices, inhalators, etc., can solve the problems of increasing the complexity and bulk of the device, unable to achieve sufficient inhalation to create such a substantial flow of air for some users, and the device delivers a bit of apical doses, etc., to achieve the effect of overcoming many deficiencies of prior art devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
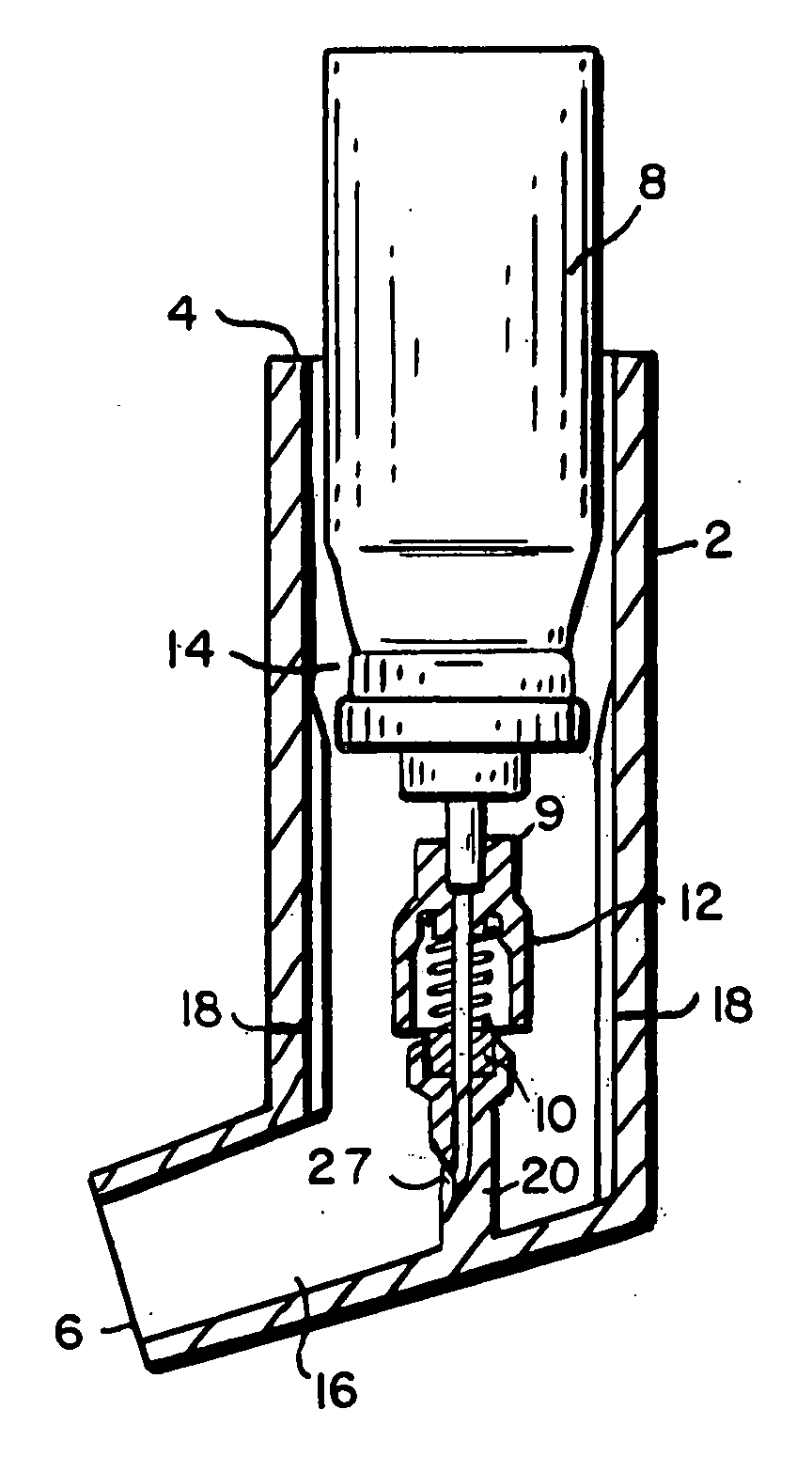
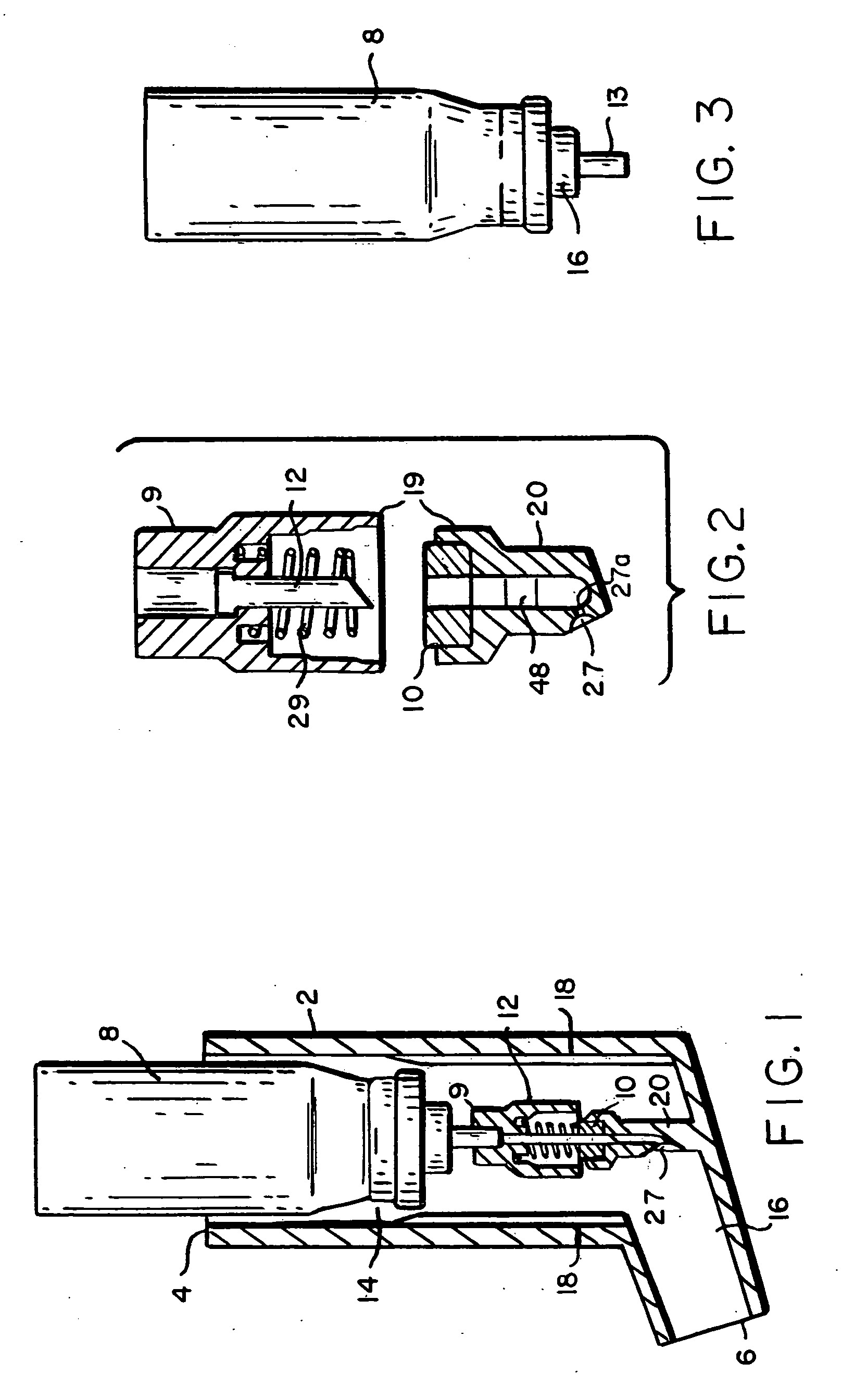
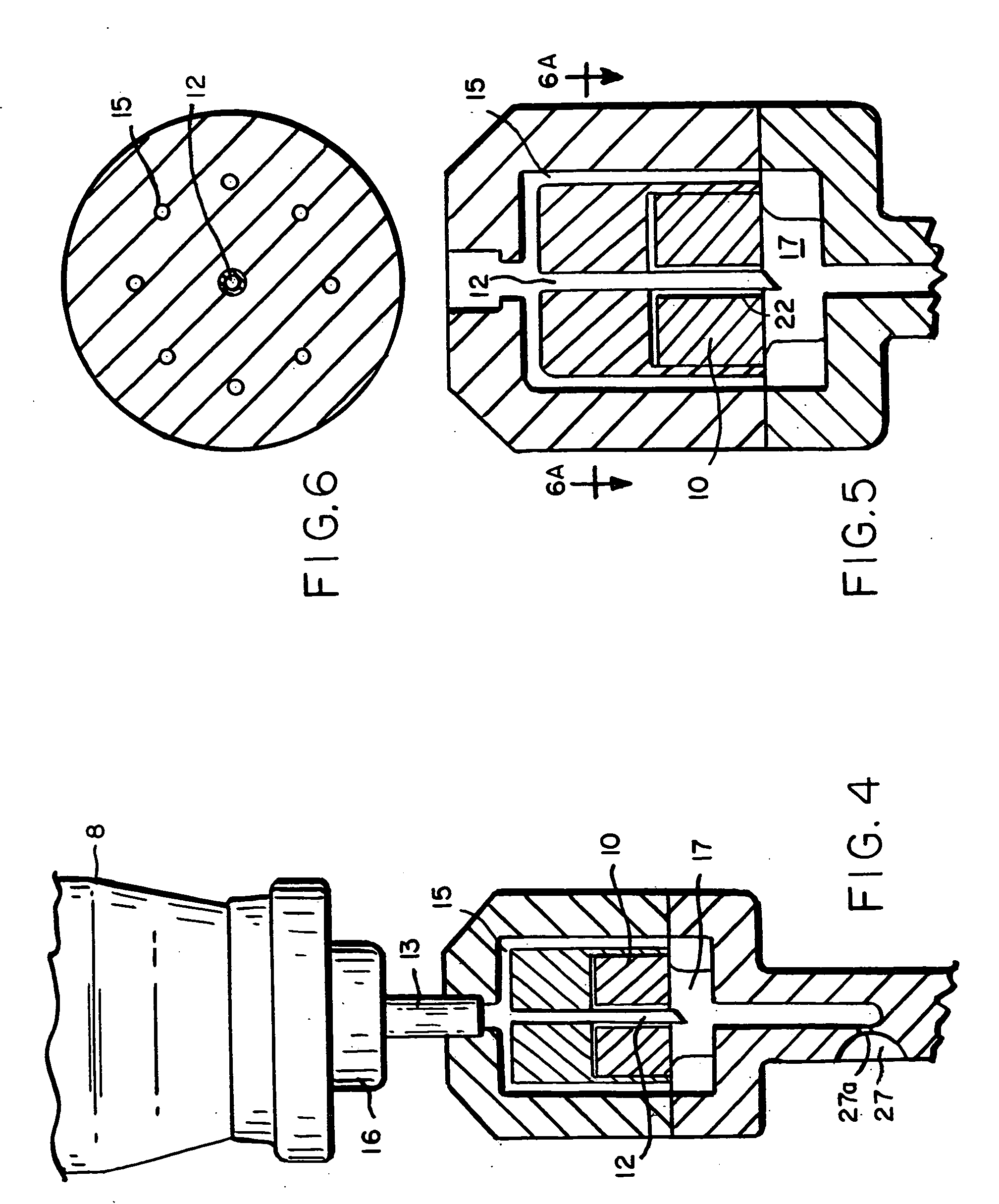
[0144] A number of tests were performed to analyze the dispersement of the propellant and agent out of the second end of the device. In these tests, the delivery device of the present invention was used to deliver the propellant with dispersed agent into a “black box” shown in FIG. 19. The black box comprises an elongate box approximately 2 feet long, 1.0 foot high and 1.0 foot wide. The black box has, at one end of its length, an opening through which the second end 6 of the device is inserted. Along the front of the black box is a short wall shielding a series of eight to ten lights from the camera lens and highlighting the powder stream discharged into the box. A camera on a tripod operating at, for example, approximately 300 frames / second, in some cases 3000 frames / second takes snapshots of the interior of the black box. The device of the present invention is actuated to dispel propellant and agent through the opening in the black box as the camera takes snapshots of the interio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com