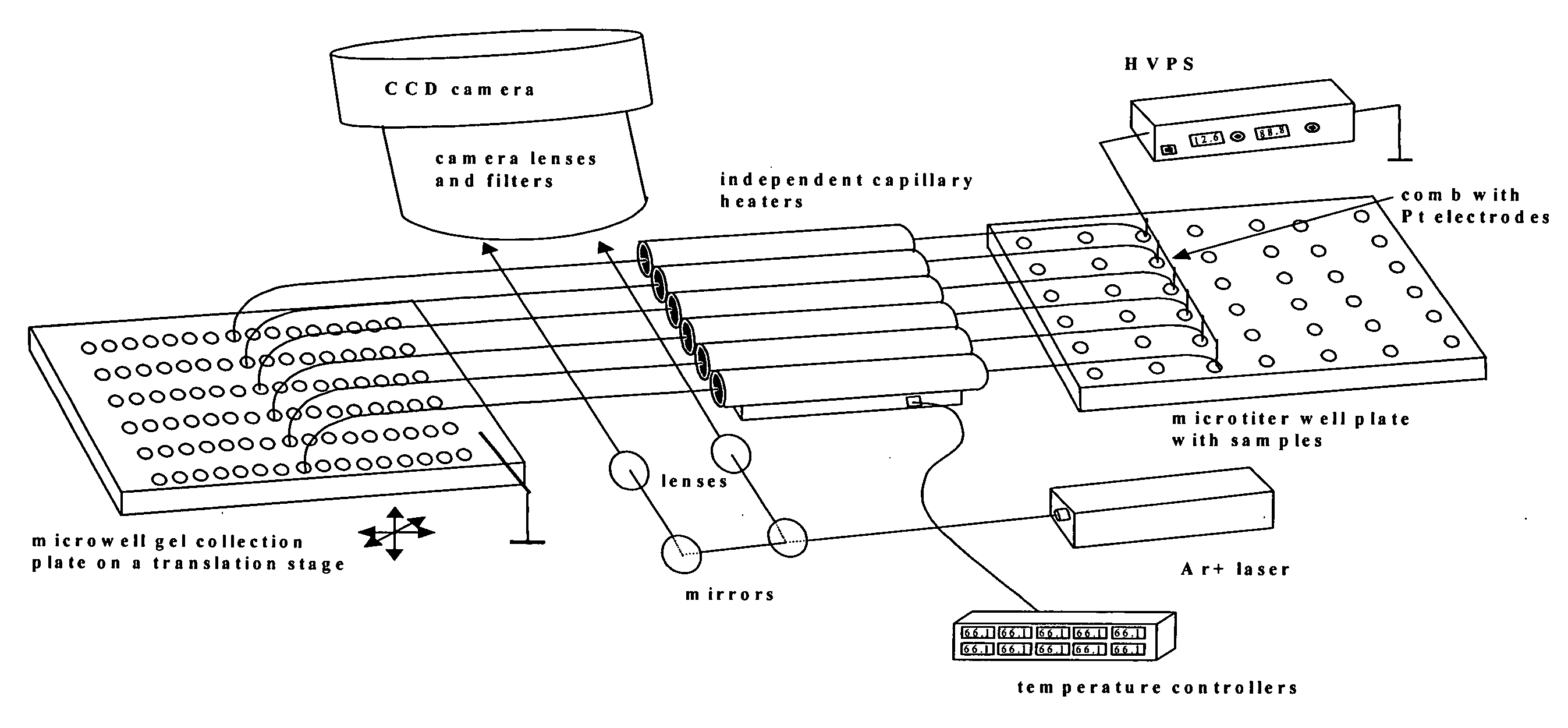
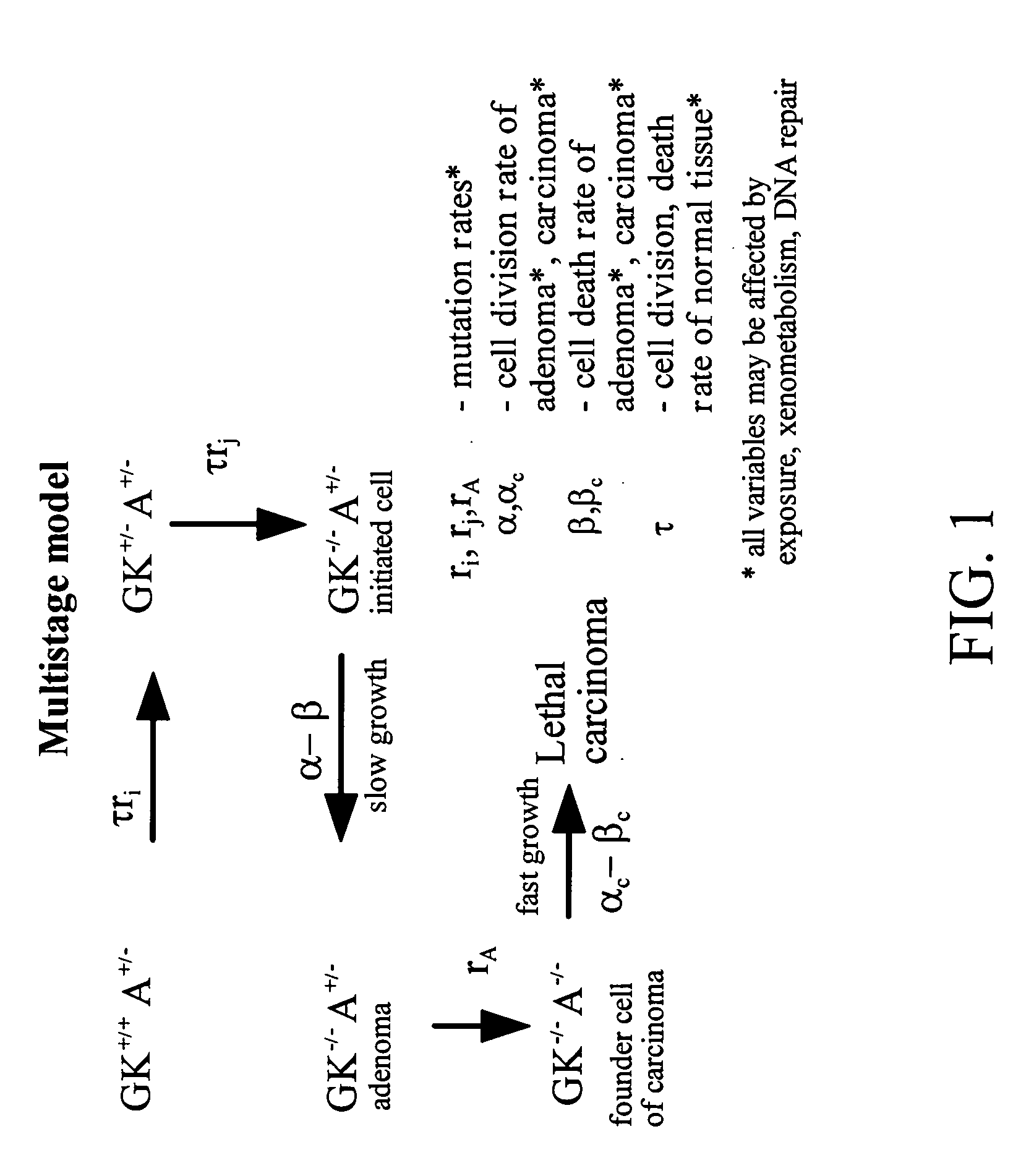
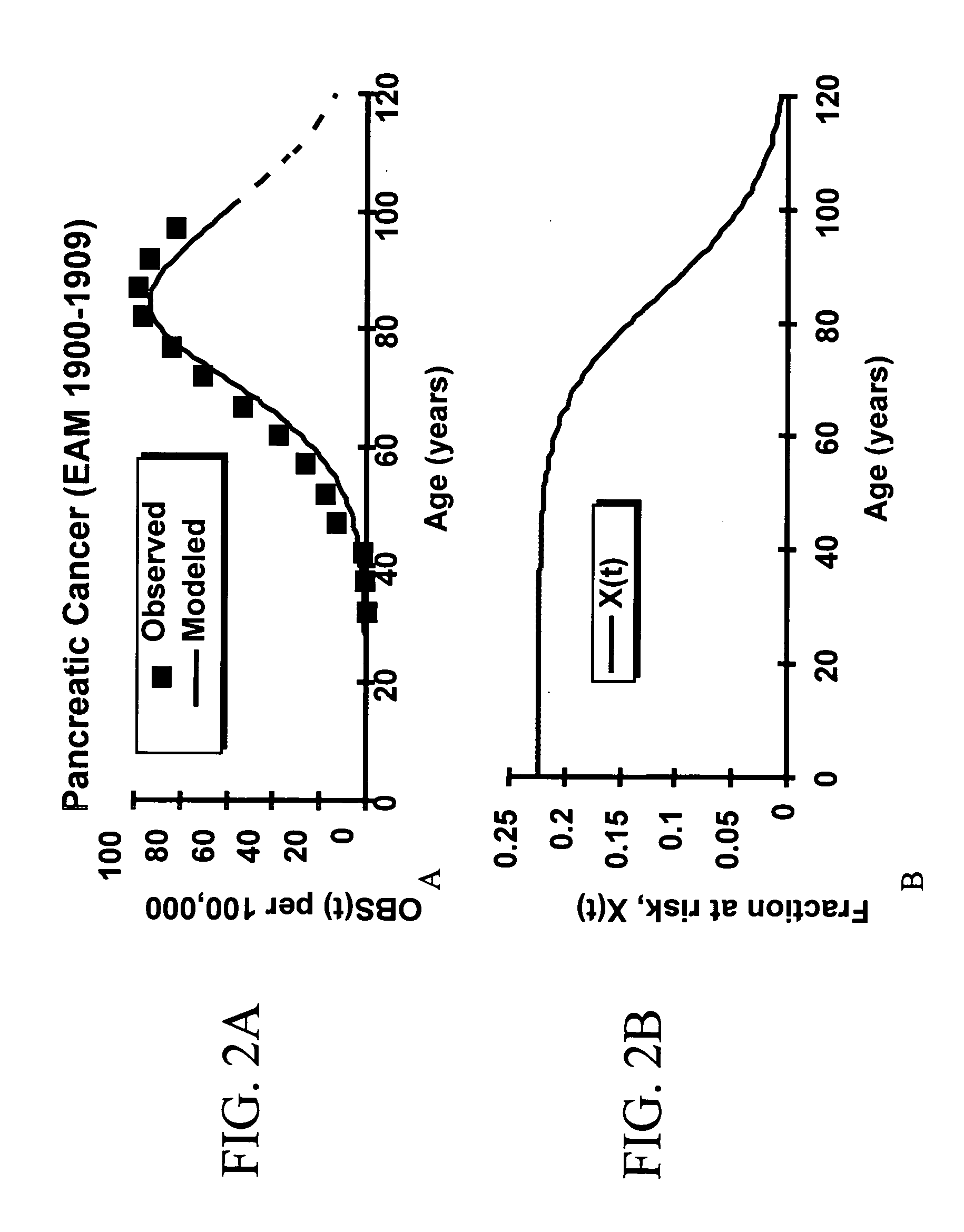
Methods of identifying point mutations in a genome that affect the risk for or the age of appearance of disease
a technology of genome and mutation, applied in the field of genome point mutation identification, can solve the problem of high cost of mutation discovery in large populations, and achieve the effect of high performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Introduction
[0089]Many inherited mutations are known to cause or predispose people to disease. As the human genome is mapped and sequenced, the number of markers that allow for genome-wide scans has increased dramatically allowing many regions linked to disease to be determined or mapped by exclusion (Nelen, M. et al., 1996. Nat. Genet. 13:114-116; Comuzzie et al., 1997. Nat. Genet. 15:273-276; Marshall, E. 1997. Science. 277:1752-1753). To date, some 1339 genes associated with human disease have been chromosomally mapped (Genome Database, Johns Hopkins University). However, when any long sequences are compared between two randomly chosen alleles in the human population, approximately two variations are found for every 1000 bp (Rowen, L. et al., 1997. Science. 278:605-607). Thus in a large human population any 1000 bp would be expected to display many sequences differing from the canonical sequence. Some of these sequence variants may affect physiological functions such as tumor sup...
case ii
Risk is Conferred by Homozygosity for a Recessive Mutation Non-Deleterious for Reproductive Fitness.
[0270]In the case where primary genetic risk for colon cancer requires inheritance of two recessive alleles of the same gene, neither of which affect reproductive fitness, the fraction of recessive homozygotes would be q2=0.4 and q=0.63 for a monogenic disorder.
[0271]Since these recessive alleles in homozygous or heterozygous form have by our definition no effect on reproductive fitness, that they might have reached so high a fraction in present day populations if the mutation rate for a single gene were about twice the average for all gene inactivating mutations or if the risk were distributed over several different genes. As in Case I, one could not logically deduce which stage of carcinogenesis might be affected by the recessive homozygous state.
Case III: Genetic Risk is Conferred by a Recessive Mutation Deleterious for Reproductive Fitness.
[0272]A third possibility is that risk is...
example 2
[0302]Ah=∫0∞OBSR(h,t)t=∫0∞Fh·(1-S(h,t))·POBS(h,t)Fh+(1-Fh)·1 / fh∫0t(1-S(h,t))·POBS(h,t)tt=∫0∞Fh·(1-S(h,t))·POBS(h,t)·-1 / fh∫0t(1-S(h,t))·POBS(h,t)tFh·-1 / fh∫0t(1-S(h,t))·POBS(h,t)t+(1-Fh)t
In order to permit integration, we introduce the variable v such that:
v=-1 / fh∫0t(1-S(h,t))·POBS(h,t)tvt=-1 / fh·(1-S(h,t))·POBS(h,t)·-1 / fh∫0t(1-S(h,t))·POBS(h,t)t
This expression is Equation 26 in context. Note that by first dividing R(h,t) from OBS(h,t), we have avoided needing to characterize R(h,t), which is itself not explicitly integrable.
The use of the maximum value of OBS*(h,t) to define Fh in terms of κh and fh.
[0303]Our third equation in our explicit derivation of the fraction at risk, Fh, came from the observation that the martality curves adjusted for survival and underreporting reached a maximum. Using Equations 19 and 21, we note that:
OBS*(h,t)=Fh·κh·(t-Δh)n-1Fh+(1-Fh)·1fh∫0t(1-S(h,t))·κh·(t-Δh)n-1tFh·κh·(t-Δh)n-1Fh+(1-Fh)·1(t)
Evaluating the derivative at age t=tmax, where the derivative of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequencies | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com