Ultra-high Yield Intravenous Immune Globulin Preparation
a technology of immune globulin and intravenous injection, which is applied in the direction of extracellular fluid disorder, drug composition, peptide, etc., can solve the problems of permanent denaturation, increase in temperature, and increase in denaturation danger, and achieves rapid infused, high yield, and greater patient tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
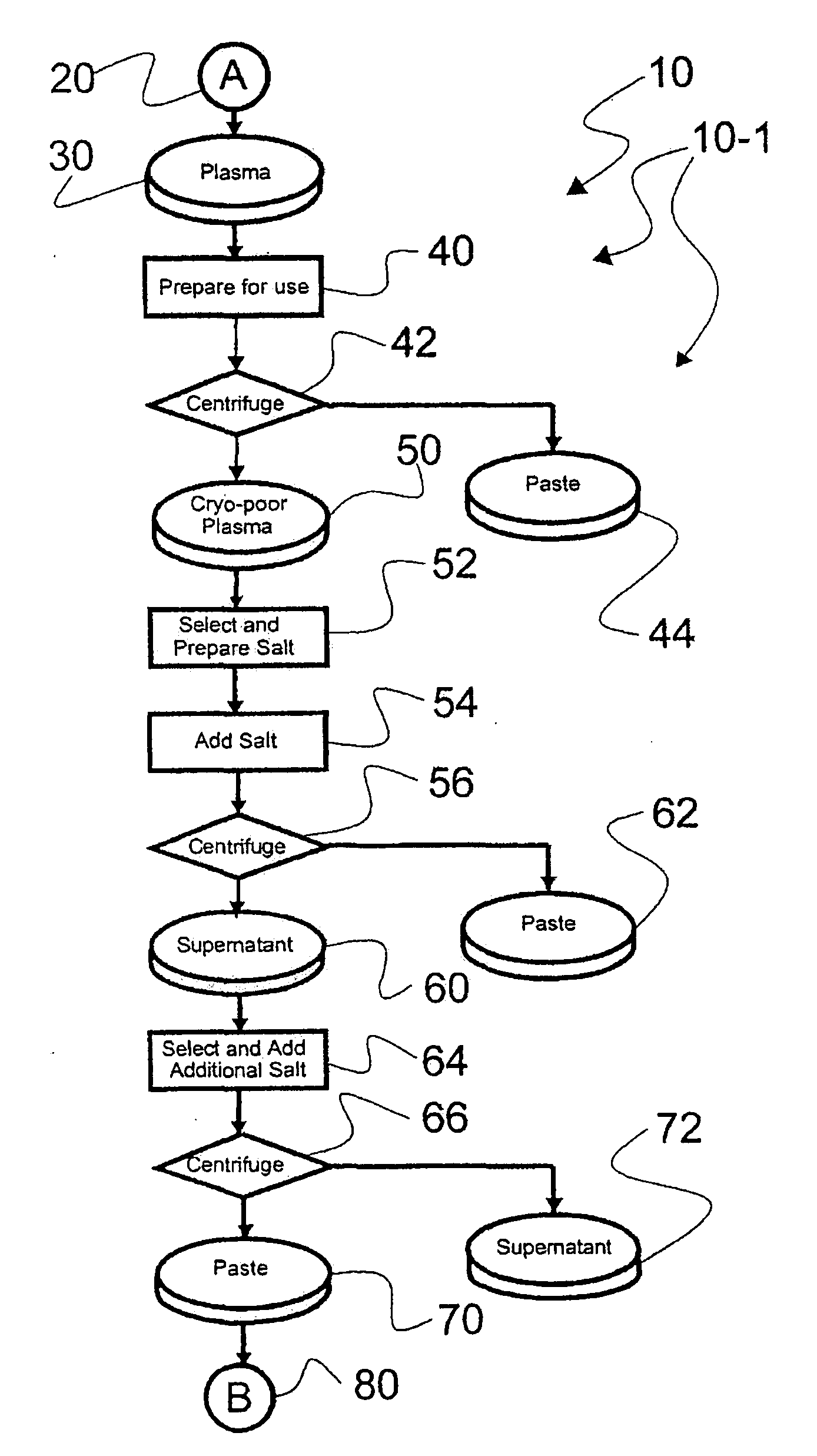
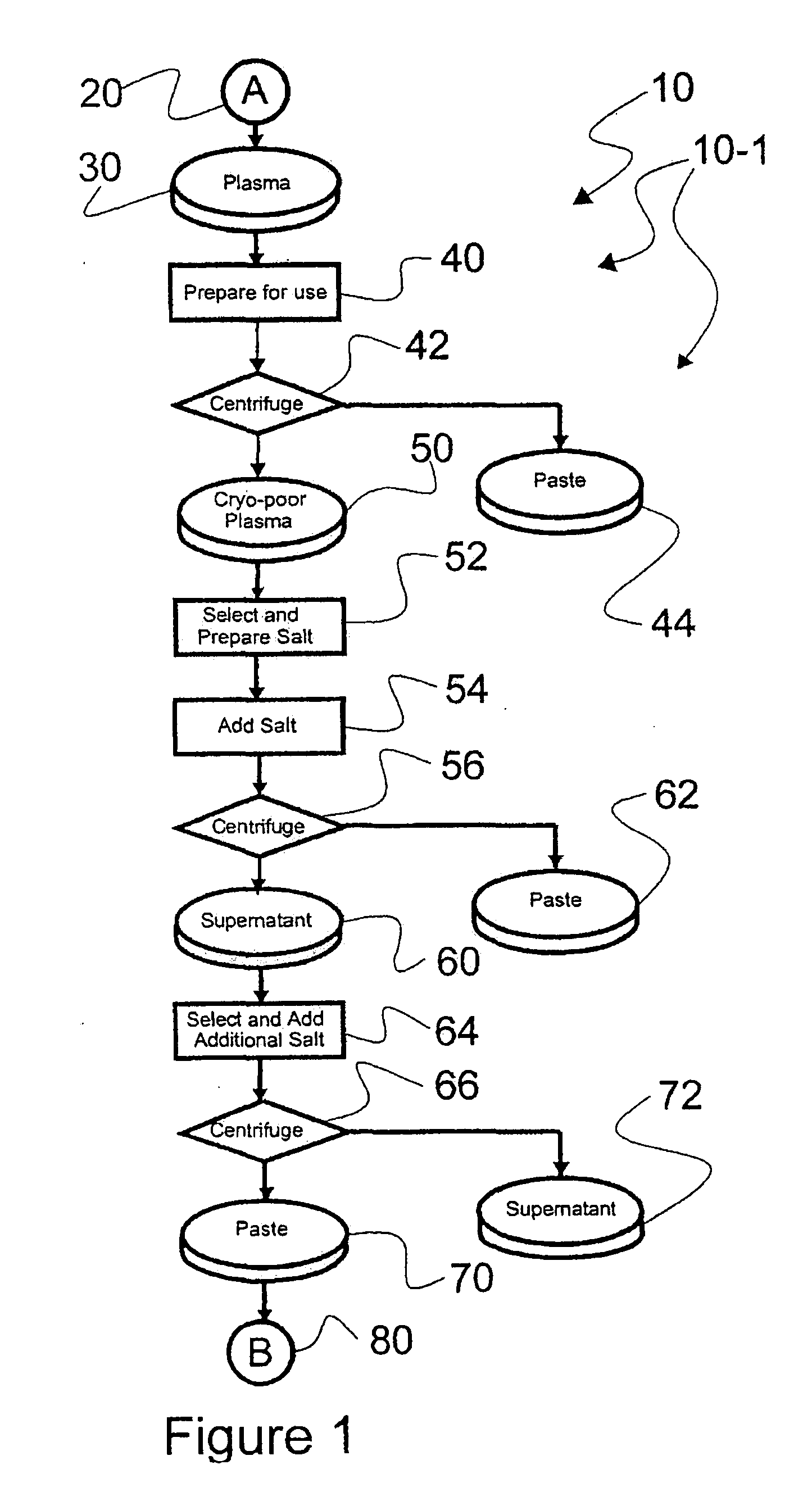
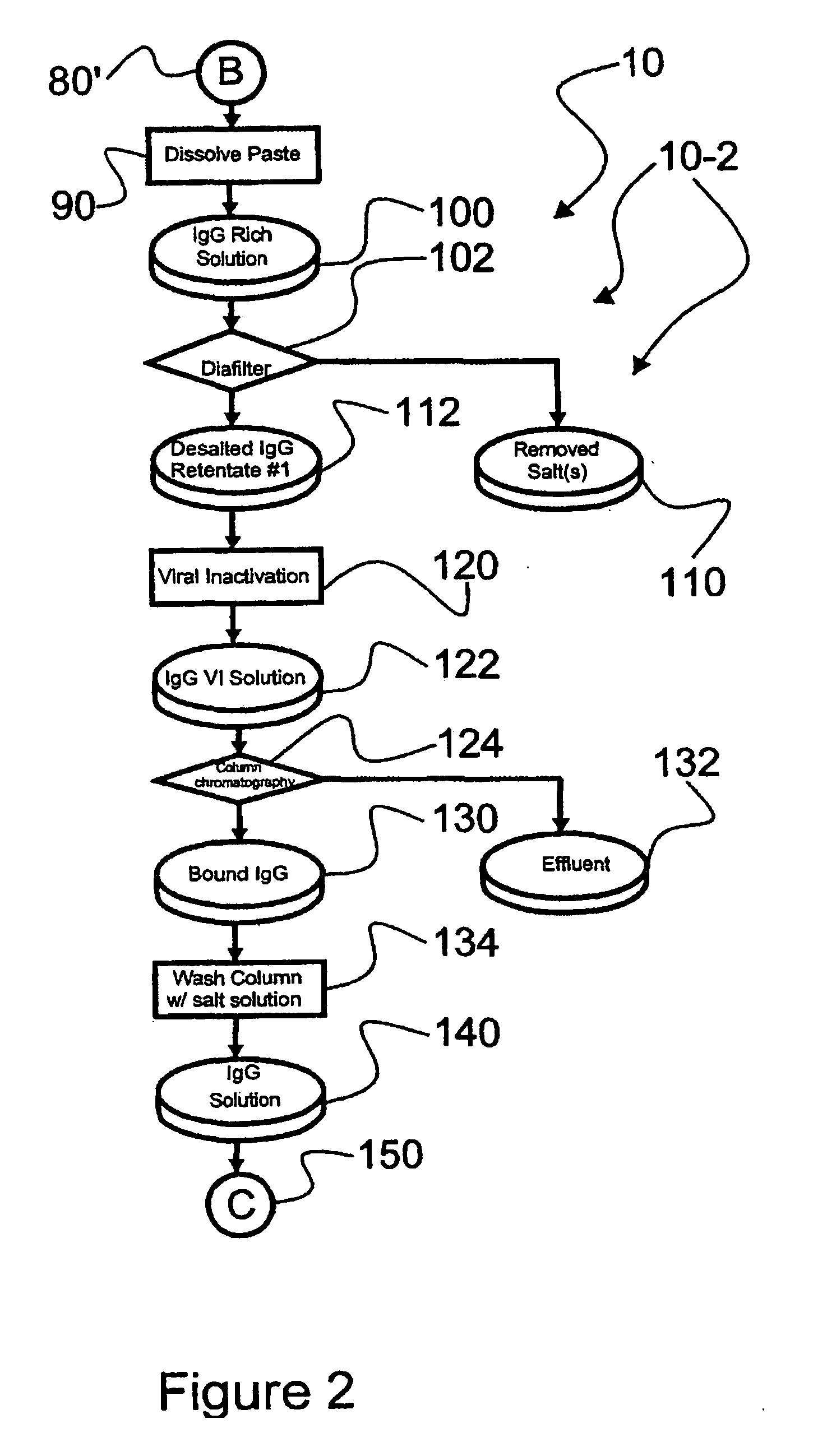
[0044]Reference is now made to flow path elements illustrated in FIGS. 1-4. Generally, each rectangular box is used to illustrate a procedural step; each diamond is used to demonstrate a separation step; each elliptical cylinder designates a product resulting from a preceding procedural or separation step; and each circle is used to identify either a starting point or an off sheet continuation path point.
[0045]Reference is now made to FIG. 1 wherein an initial portion 10-1 of an preferred IgG process flow path, generally numbered 10, is seen. As indicated after initial starting point 20, a volume of plasma 30 to be processed is selected for processing. It should be noted that while plasma 30 is used by example in this description of an illustrated embodiment, other blood-based products may be processed within the scope of the instant invention. Also, after preparation for use, a separation step 42 is used to separate a paste 44 from prepared cryo-poor plasma 50, as is disclosed in m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com