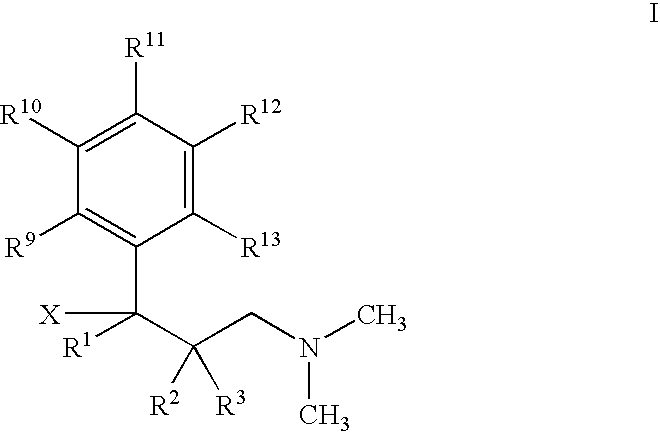
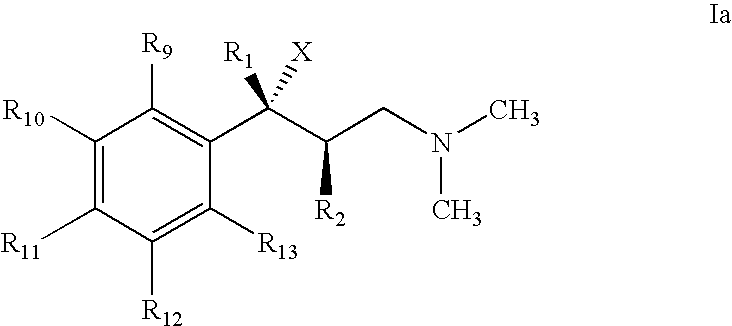
Use of 1-Phenyl-3-Dimethylamino-propane Compounds for Treating Neuropathic Pain
a technology of dimethylaminopropane and neuropathic pain, which is applied in the field of use of 1phenyl3dimethylaminopropane compounds, can solve the problems of not being effective at all, not being easy to achieve, and only being slightly effective, so as to achieve high effective treatment of neuropathic pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 0
Tested Substances
[0089]The following compounds were tested and are hereinafter correspondingly abbreviated as compound (or Comp.) 1, etc. in Table 1:
TABLE 1Name:Compound(2RS,3RS)-1-dimethylamino-3-(3-methoxyphenyl)-2-1methylpentan-3-ol: hydrochloride(2S,3S)-1-dimethylamino-3-(3-methoxyphenyl)-2-2methylpentan-3-ol; hydrochloride(2R,3R)-1-dimethylamino-3-(3-methoxyphenyl)-2-3methylpentan-3-ol; hydrochloride(1RS,2RS)-3-(3-dimethylamino-1-hydroxy-1,2-5dimethylpropyl)-phenol; hydrochloride(1S,2S)-3-(3-dimethylamino-1-hydroxy-1,2-6dimethylpropyl)phenol; hydrochloride(1R,2R)-3-(3-dimethylamino-1-hydroxy-1,2-7dimethylpropyl)phenol; hydrochloride(2RS,3RS)-3-(difluoromethylphenyl)-1-dimethylamino-2-8methylpentan-3-ol; hydrochloride(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methylpropyl)-9phenol; hydrochloride(1S,2S)-3-(3-dimethylamino-1-ethyl-2-10methylpropyl)phenol; hydrochloride3-(3-dimethylamino-1,2-dimethylpropenyl)-phenol;14hydrochlorideAdditionally:MorphineMorGabapentinGBP
example 1
Bennett
Neuropathic Pain in Rats
[0090]The effectiveness in treating neuropathic pain was investigated in the Bennett model (chronic constriction injury; Bennett and Xie, 1988, Pain 33: 87-107).
[0091]Sprague-Dawley rats weighing 140-160 g are provided with four loose ligatures of the right sciatic nerve under nembutal narcosis. The animals develop a hypersensitivity in the paw innervated by the damaged nerve, which after a healing phase of one week is quantified over about four weeks by means of a 4° C. cold metal plate (cold allodynia). The animals are observed for two minutes on this plate and the number of retractive movements of the damaged paw are measured. The effect of the substance is determined with reference to the base value before application of the substance, at four different times over a period of one hour(15, 30, 45 and 60 minutes after application) and the resultant area under the curve (AUD) as well as the inhibition of cold allodynia at the individual measurement po...
example 2
Chung
In Vivo Experiments According to Chung
[0093]Spinal nerve ligatures according to Kim & Chung (1992 Pain 50, 355-363) were applied to the left L5 / L6 spinal nerves of male Spraque-Dawley rats. Four to six days after the operation the tactile threshold baseline (withdrawal threshold) was measured on the ipsilateral and contralateral rear paw by an electronic von Frey anaesthesiometer (IITC Life Science, USA). After the test and measurement of the baseline, morphine, gabapentin and some of the aforementioned compounds used according to the invention were administered. The tactile withdrawal thresholds were measured 30 minutes after the administration. The results are expressed as ED50 and % maximal possible effect (% MPE) on the ipsilateral side, in which the baseline is taken as 0% and the withdrawal threshold of a control group is taken as 100% MPE.
[0094]The results together with those from Example 1 are summarized in Table 2:
TABLE 2Testing of the inhibition of neuropathic pain in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| withdrawal threshold | aaaaa | aaaaa |
| withdrawal threshold | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com