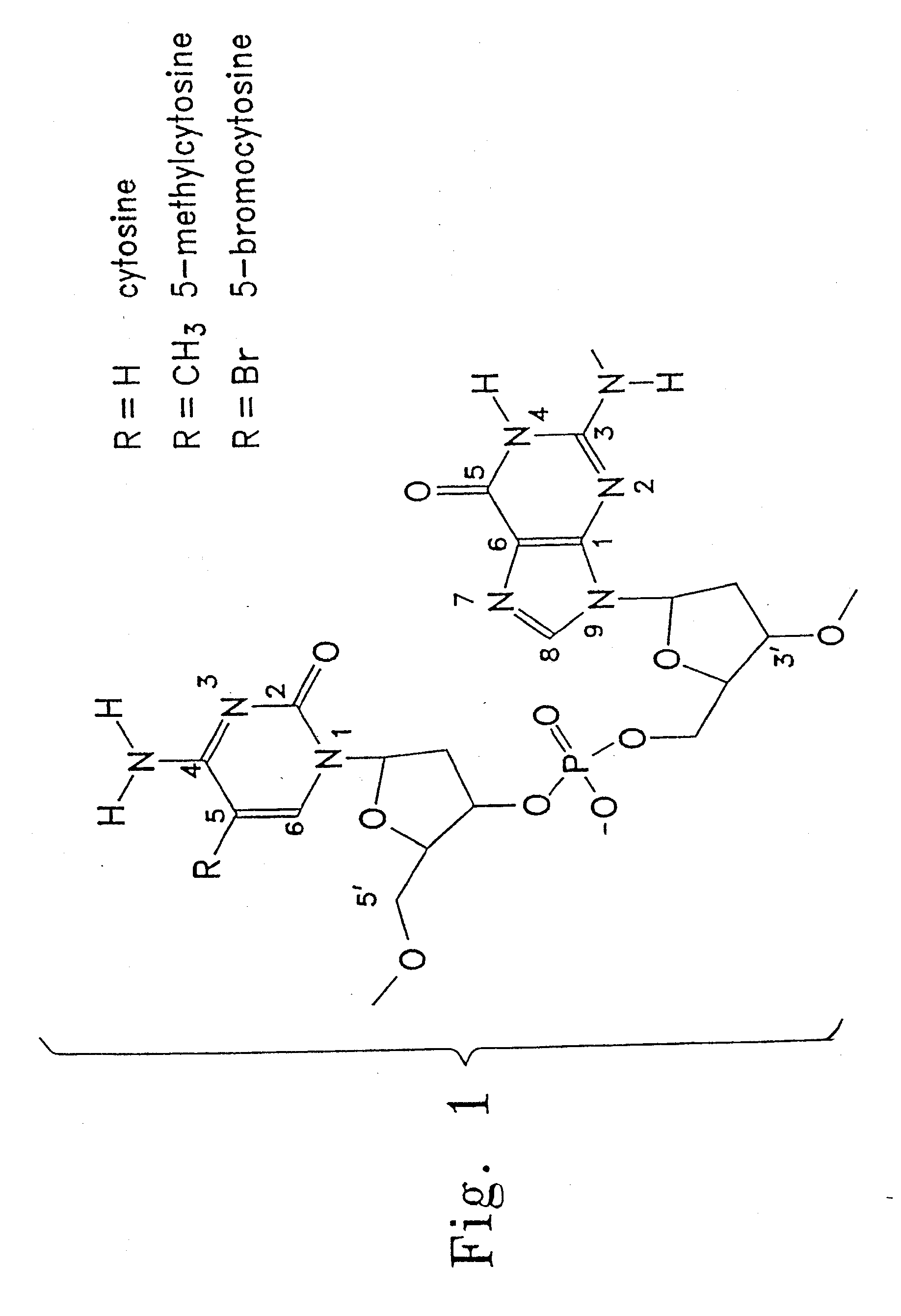
Immunostimulatory oligonucleotides with modified bases and methods of use thereof
a technology of immunomodulatory oligonucleotides and bases, applied in the field of immunomodulatory compositions, can solve the problems of not always eliciting the immune response necessary to achieve a therapeutic effect, failure to elicit cellular responses, anaphylactic shock,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Stimulation of Cytokine Production by Oligonucleotides Comprising Modified ISS
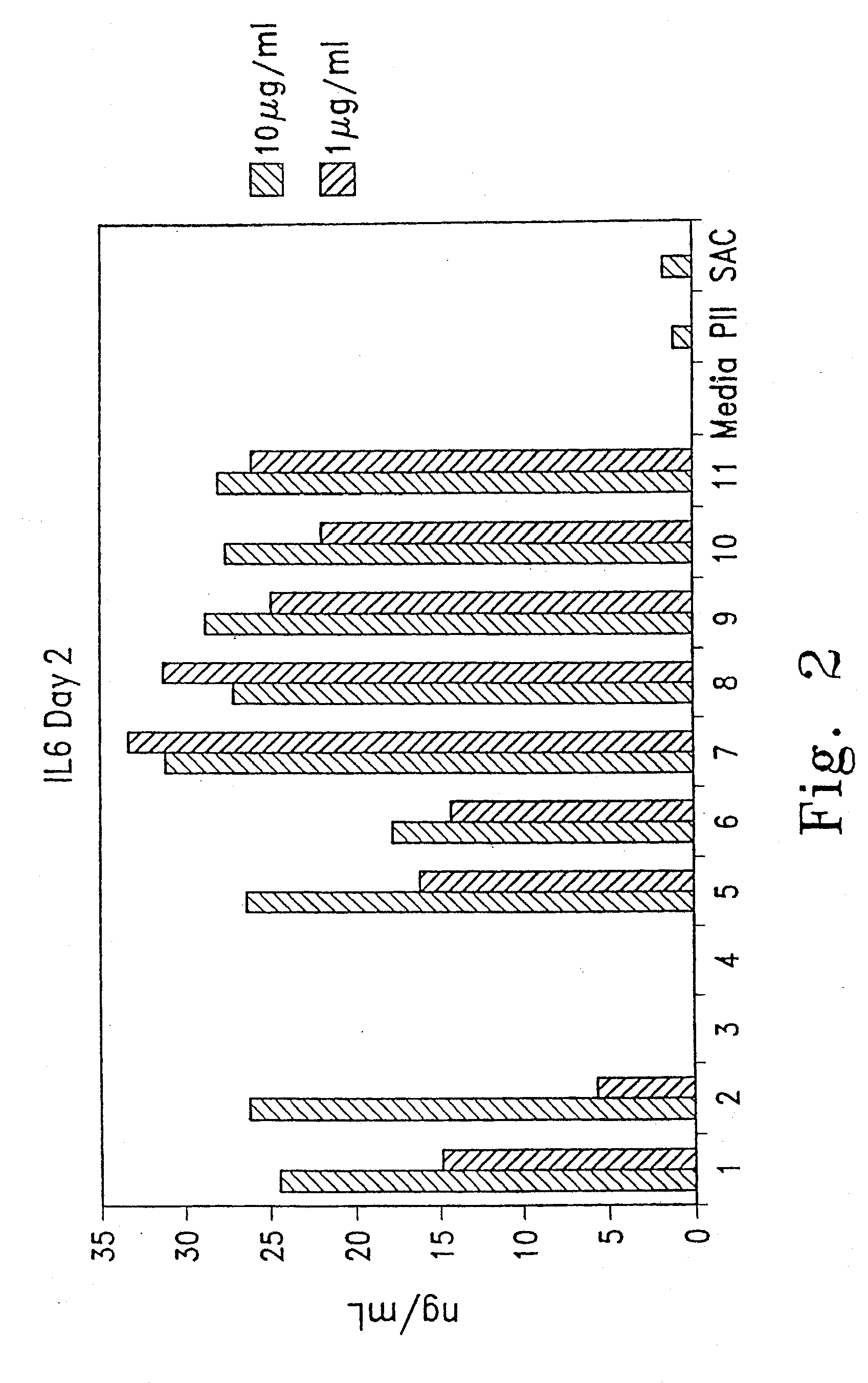
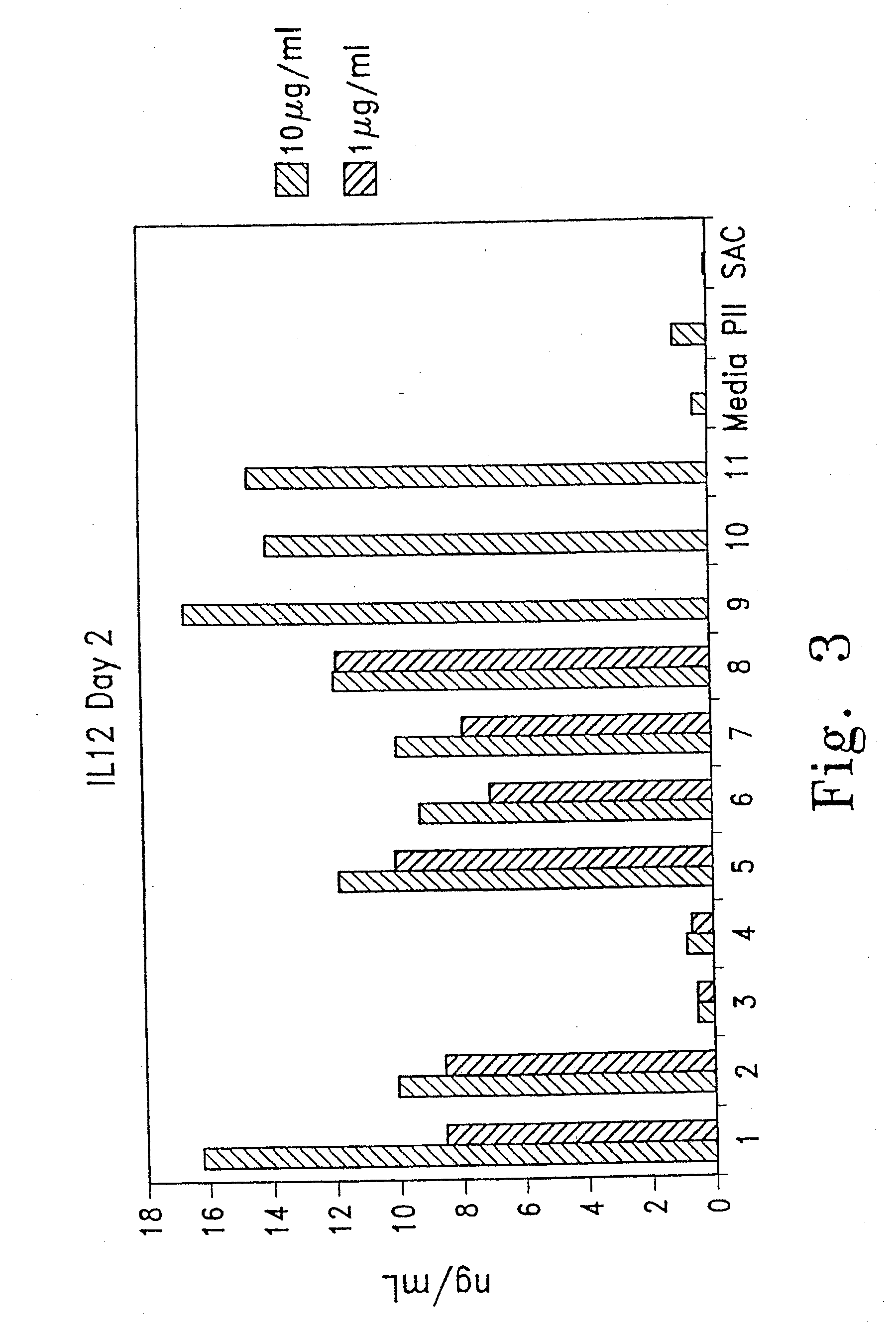
[0146]Several oligonucleotides comprising modified ISS were tested for their immunostimulatory activity on mouse splenocytes and on human peripheral blood mononuclear cells (hPBMCs). Immunostimulation in response to oligonucleotide was assessed by measurement of cytokine secretion into the culture media and by cell proliferation. Cytokine levels in the culture supernatant were determined by enzyme-linked immunosorbent assay (ELISA) tests.
[0147]The oligonucleotides were synthesized using standard solid phase oligonucleotide techniques. The solid phase ready analog monomers were purchased from Glen Research, Sterling, Va. and included in the standard manner in a solid phase oligonucleotide synthesizer. The synthesis of the oligonucleotides were performed by TriLink BioTechnologies Inc., San Diego, Calif.
[0148]Cells were isolated and prepared using standard techniques. hPBMCs were isolated from heparinized pe...
example 2
Potentiation of an Immune Response with Adjuvant Co-Administration
[0152]The effect of adjuvant co-administration with antigen and modified ISS (mISS) on an immune response to the antigen is examined using the adjuvants alum and MF59. Compositions comprising 1 μg AgE, a major allergic component is short ragweed, is injected intradermally into mice at week 0, 2, and 4. Antigen compositions usable are listed below:
AgEAgE-mISS conjugateAgE + mISS mix (equivalent)AgE + mISS mix (50 μg mISS)AgE and MF59AgE-mISS conjugate and MF59AgE and alum (25 μg)AgE-mISS conjugate and alum (25 μg)AgE and alum (800 μg)
[0153]The amount of anti-AgE antibody in the serum of the mice is determined at day 0 and weeks 2, 4, and 6. Anti-AgE antibody assays (IgE, IgG1, IgG2a) are performed by ELISA tests using the original AgE vaccine as the coated antigen on microtiter plates as described in Raz et al. (1996).
[0154]A comparison of anti-AgE antibody production, including anti-AgE antibody subtypes, provides an ...
example 3
Selective Induction of a Th1-Type Response in a Host after Administration of a Composition Comprising a Modified ISS
[0155]In mice, IgG2A antibodies are serological markers for a Th1-type immune response, whereas IgG1 antibodies are indicative of a Th2-type immune response. The production of the cytokine IFN-γ is also an indicator of a Th1-type response.
[0156]To determine which response, if any, would be produced by mice who received modified ISS compositions according to the invention, groups of BALB / c mice are immunized with 10 μg β-galactosidase (β-Gal) protein. Some mice receive β-Gal alone, some receive a modified ISS-β-Gal conjugate, some receive a modified ISS-β-Gal-adjuvant composition, and some receive a composition of β-Gal with a nonstimulatory oligonucleotide. Naïve mice are also included in the experiment.
[0157]At two week intervals, any IgG2A and IgG1 to β-Gal present in the serum of each mouse is measured by ELISA on microtiter pates coated with β-Gal. The titers of an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com