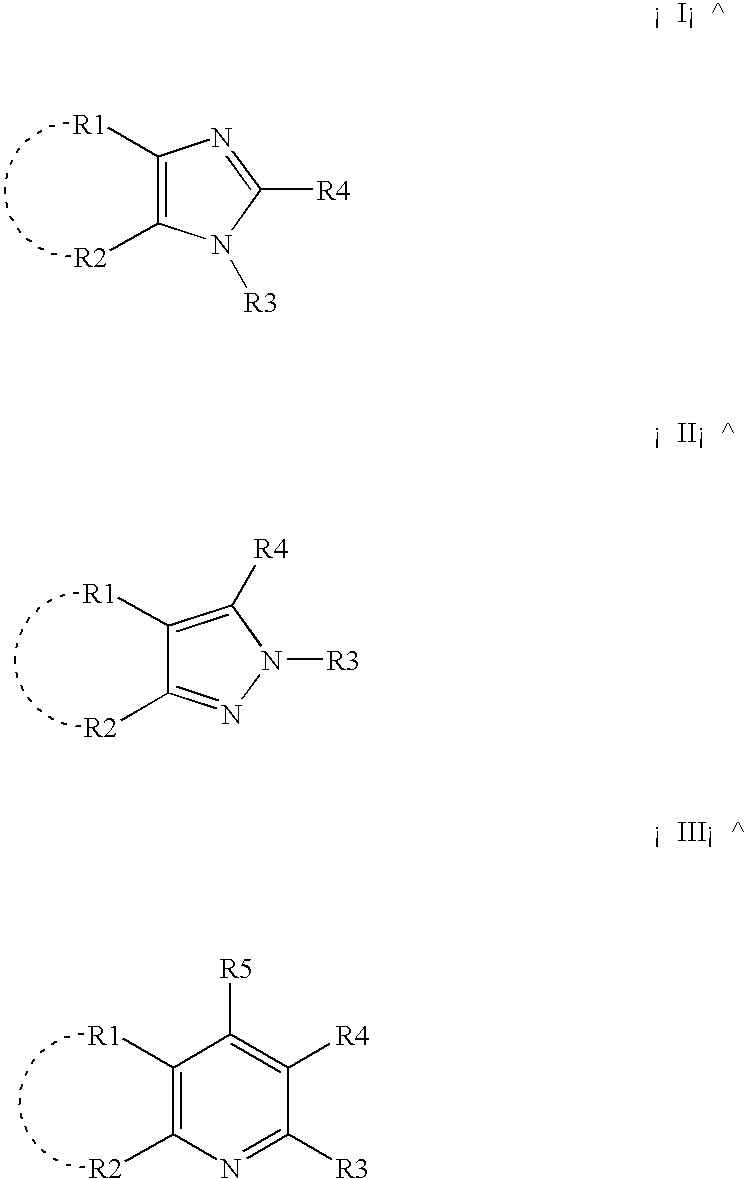
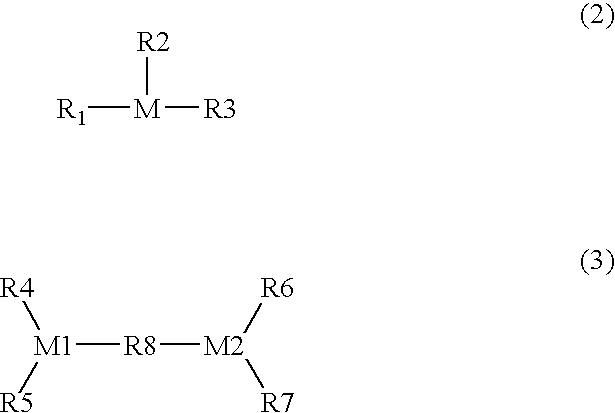
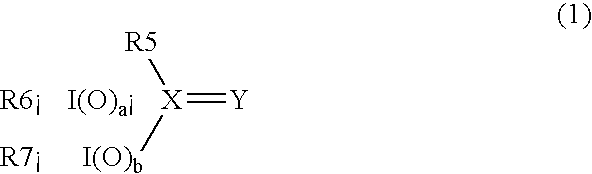Process for producing carboxylic acid anhydrides
a carboxylic acid anhydride and carboxylic acid technology, applied in the field of process for producing carboxylic acid anhydrides, can solve the problems of reducing the efficiency of heterocyclic aromatic compounds, so as to achieve effective stabilization of rhodium catalysts and high reaction rates. , the effect of increasing the reaction ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples [UNK]
EXAMPLES 12˜14
[0041](Influence of Added Amount of Organic Promoters on Reaction Rate)
[0042]The carbonylation reactions were carried out under the same conditions as the Comparative Example 1, except that 2 wt. %, 4 wt. % and 6 wt. % of organic promoters, N-acetylimidazole, were added in the reaction media. The experimental results were recorded in Table 4. It is obvious from Table 4 that the STY values of the carbonylation reaction were increased synchronously with the increase in the added amount of organic promoter, which shows the carbonylation reaction rate can indeed be satisfactorily increased by the increase in the added amount of these kinds of organic promoters according to the present invention. In addition, when the Example 14 is compared with the Comparative Example 1, it is found that the addition of these kinds of organic promoters can maintain the original reaction rate with the added amount of lithium iodide reduced, which shows these kinds of organic promoters and l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com