Novel benzamidine compound
a benzamidine and compound technology, applied in the field of new benzamidine compounds, can solve the problems of high risk, difficult hemostasis, and high risk of creating a propensity to hemorrhage, and achieve the effect of preventing thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
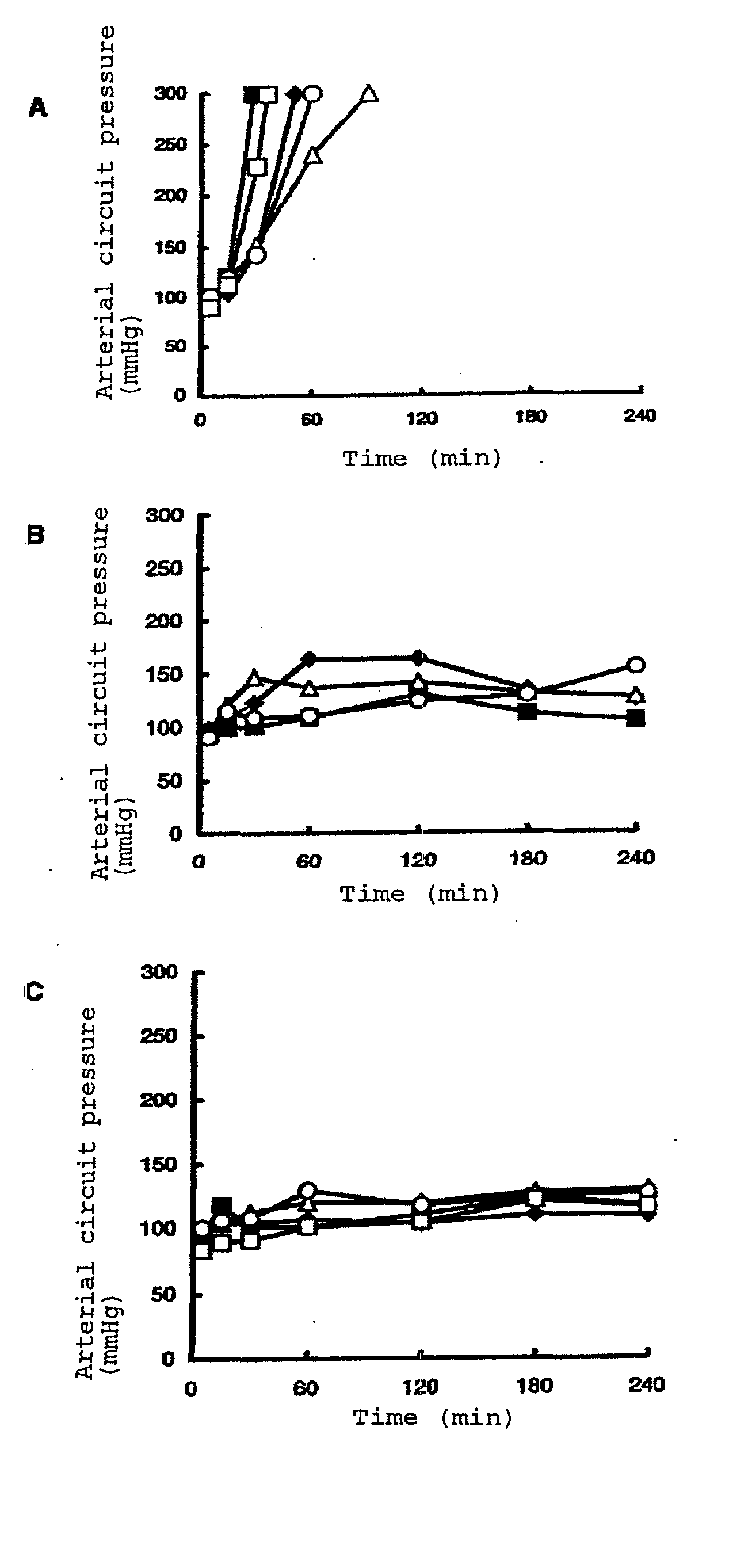
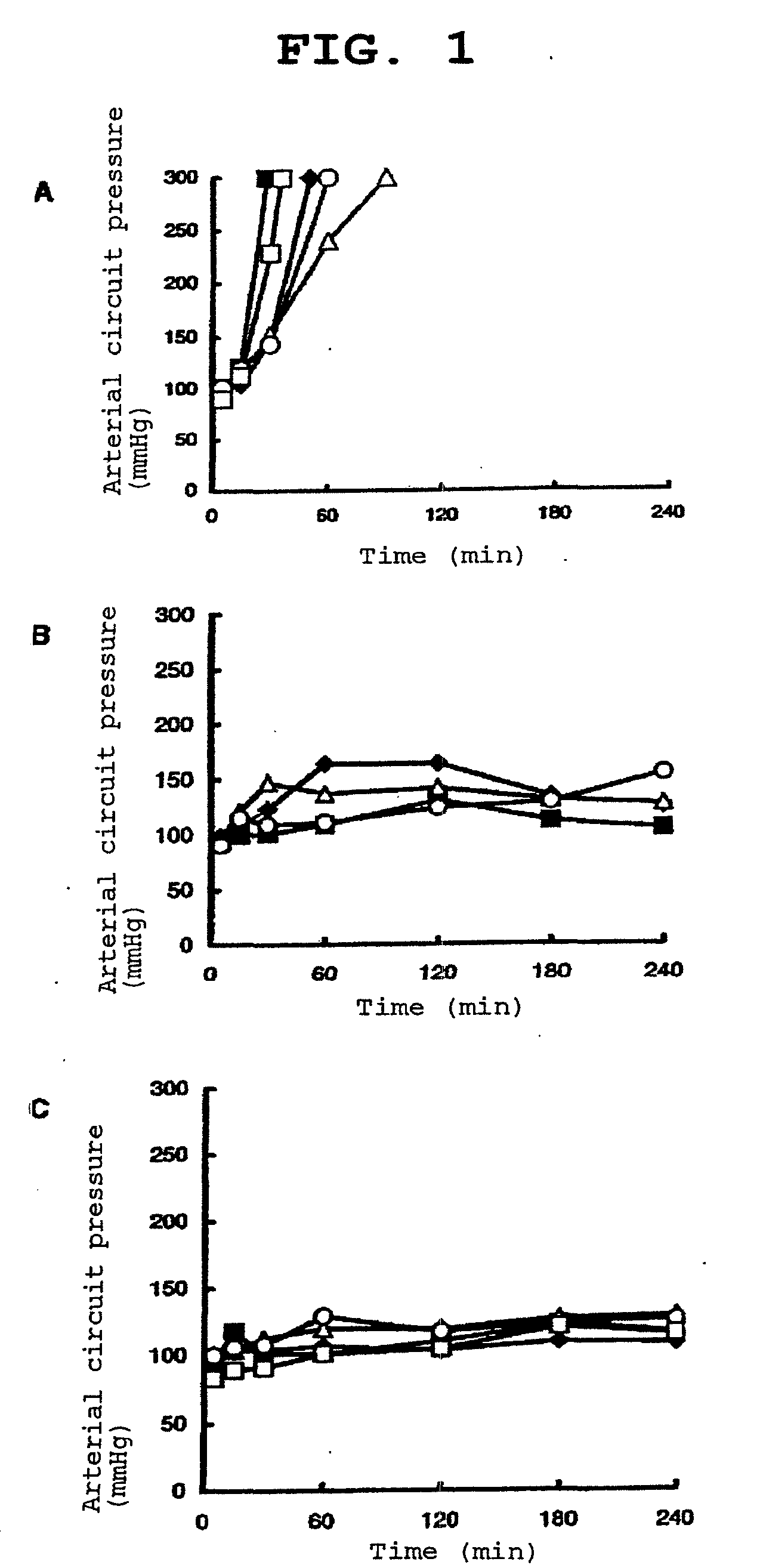
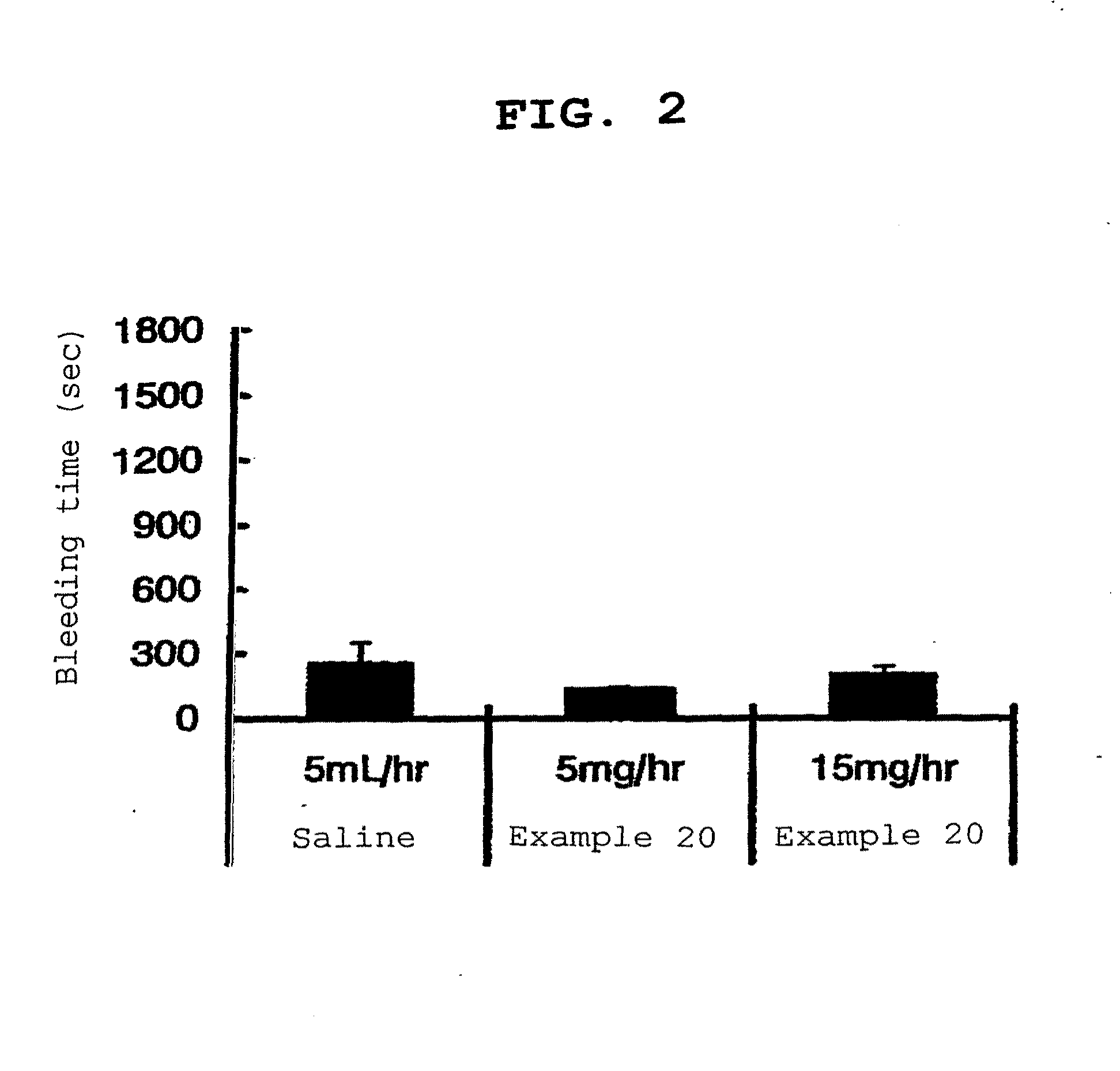
Image
Examples
example 1
2-{3-amidinophenoxy}ethyl 1-pyridin-4-ylpiperidine-4-carboxylate ditrifluoroacetate
Step 1. Synthesis of 2-(3-cyanophenoxy)ethyl acetate
[0170] 3-Cyanophenol (10.1 g, 84.8 mmol) and potassium carbonate (19.5 g, 141 mmol) were suspended in acetone (280 ml), 2-bromoethyl acetate (7.8 ml, 70.7 mmol) was added, and the mixture was stirred at 50° C. for 8 hours. Sodium iodide (1.06 g, 7.07 mmol) was added to the reaction mixture, and the mixture was heated under reflux for two nights and concentrated under reduced pressure. The residue was diluted with ethyl acetate, and the mixture was washed with 1N sodium hydroxide, 1N hydrochloric acid, and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give the title compound without purification.
[0171] yield 8.94 g (43.6 mmol, 51%)
[0172]1H-NMR (CDCl3) δ 2.11 (3H, s), 4.20 (2H, br), 4.44 (2H, br), 7.14-7.16 (1H, m), 7.26-7.28 (1H, m), 7.36-7.41 (1H, m).
Step 2. Synthesis of 3-(2-hydro...
example 2
2-{3-amidinophenoxy}ethyl 4-[imino(pyrrolidin-1-yl)methyl]benzoate ditrifluoroacetate
Step 1. Synthesis of 3-hydroxybenzamidine trifluoroacetate
[0187] To 3-cyanophenol (5.00 g, 42.0 mmol) were added anhydrous ethanol (6.1 ml, 210 mmol) and 4N hydrochloric acid / 1,4-dioxane solution (55 ml), and the mixture was stirred in a closed system at room temperature for three nights. The solvent was evaporated under reduced pressure, and the obtained residue was added slowly at −78° C. to ethanol (210 ml) into which ammonia gas had been blown at the same temperature for 30 minutes. The temperature was gradually raised to room temperature, and the mixture was stirred overnight. The solvent was evaporated under reduced pressure, diethyl ether and ethanol were added to the residue, and the precipitated crystals (5.89 g) were collected by filtration. 2 g thereof was purified by reversed-phase HPLC in the same manner as in Step 4 of Example 1 to give the title compound.
[0188] yield 964 mg (3.85 m...
example 3
2-{3-amidinophenoxy}ethyl 4-({N-methyl-N-[2-methylaminoethyl]-amino}carbonyl)benzoate ditrifluoroacetate
Step 1. Synthesis of 2-{4-[(2-bromoethoxy)carbonyl]phenyl}-1,3-dimethyl-4,5-dihydro-1H-imidazo-3-lium trifluoroacetate
[0203] 4-Cyanobenzoic acid (10.1 g, 68.9 mmol) was dissolved in anhydrous ethanol (10 ml) and 4N hydrochloric acid / 1,4-dioxane (100 ml), and the mixture was stirred in a closed system at room temperature for two nights. The solvent was evaporated under reduced pressure, and the obtained residue was suspended in anhydrous ethanol (50 ml). N,N′-Dimethylethylenediamine (7.4 ml, 68.9 mmol) was added, and the mixture was stirred at room temperature overnight. The solvent was evaporated under reduced pressure, and the obtained residue (500 mg, 1.77 mmol) was dissolved in 2-bromoethanol (3 ml). p-Toluenesulfonic acid monohydrate (5 mg) was added, and the mixture was stirred at 50° C. for 2 days. The temperature was raised to 70° C., and the mixture was further stirred o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com