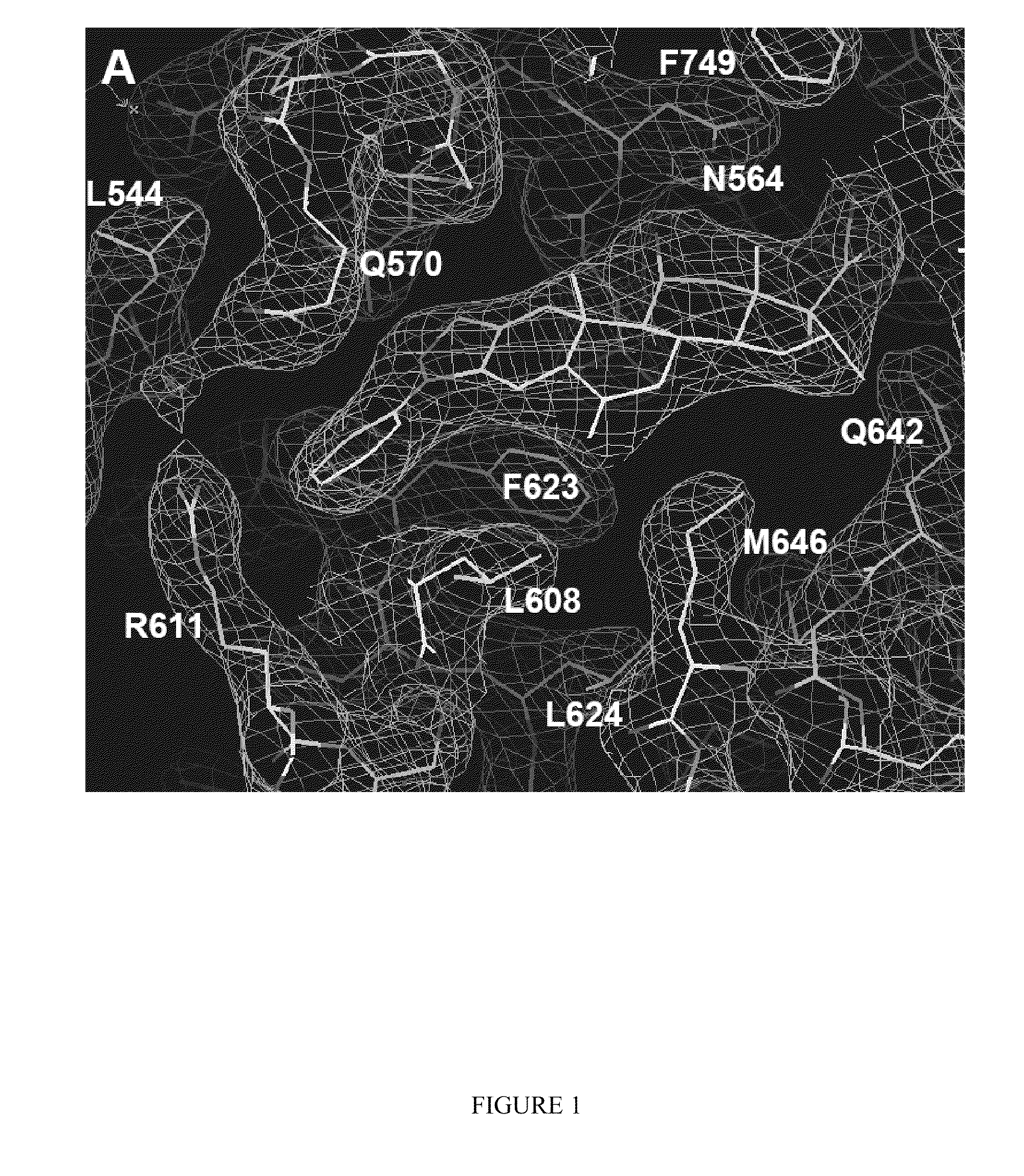
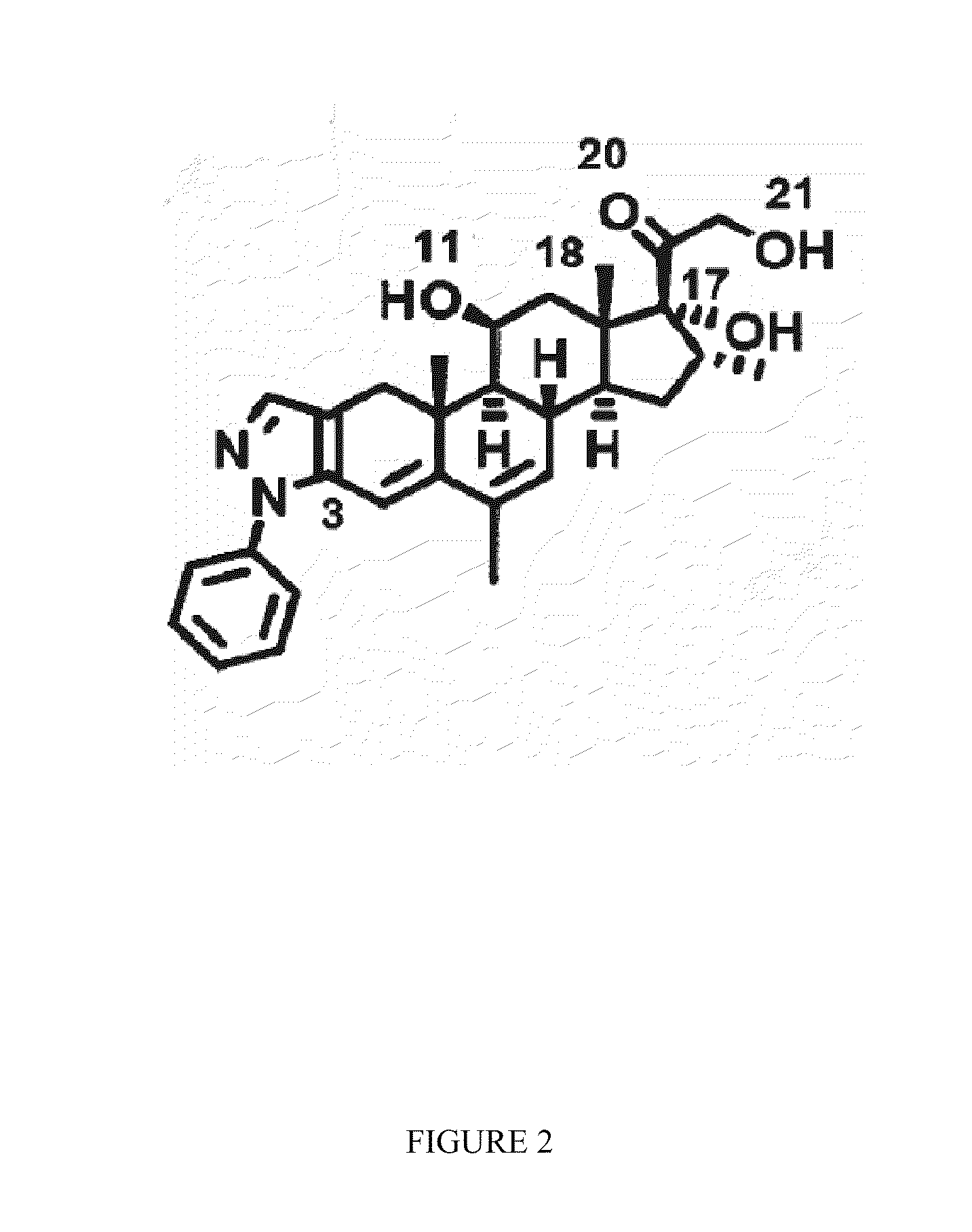
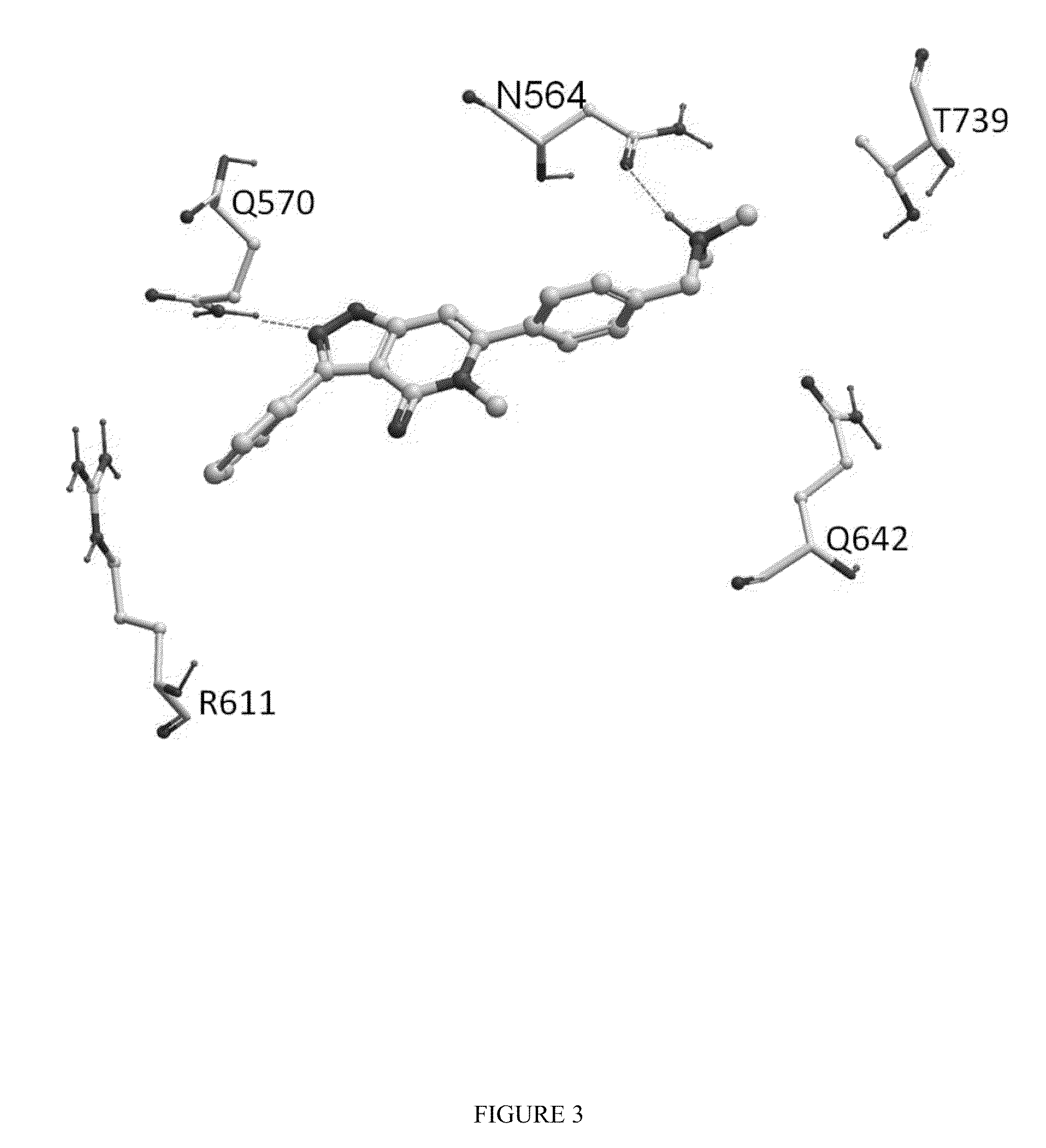
Methods of using substituted isoxazolo pyridinones as dissociated glucocorticoids
a technology of isoxazolo pyridinone and dissociated glucocorticoids, which is applied in the field of glucocorticoid receptors, can solve the problems of side effects, diabetes, osteoporosis, and significant transactivation activity, and achieves significant transactivation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
GR Transactivation on MMTV in∘AD293 Cells Transfected with 0.5 ng GR
[0090]AD293 cells were transfected with 0.5 ng GR, 100 ng pHHLuc reporter together with 5 ng Renilla control plasmids, then induced by EtOH (vehicle control), Dexamethasone (10 nM), Compound #53 (5 μM), AL438 (1 μM) and DMSO (vehicle control). Dual-Luciferase values were measured by Promega standard manual, Compound #53's transactivation activity on MMTV in AD293 cells is shown in FIG. 5.
example 2
GR Transrepression of AP1 in AD293 Cells Transfected with 10 ng GR
[0091]AD293 cells were transfected with 10 ng GR, 100 ng AP1-Luc reporter together with 5 ng Renilla control plasmids, then induced by EtOH (vehicle control), Dexamethasone (10 nM), Compound #53 (5 μM), AL438 (1 μM). Dual-Luciferase values were measured by Promega standard manual. Compound #53's GR transrepression activity on AP1 in AD293 cells is shown in FIG. 6.
example 3
GR Transpression of AP 1 in AD293 Cells Transfected with 50 ng GR
[0092]AD293 cells were transfected with 50 ng GR, 100 ng AP1-Luc reporter together with 5 ng Renilla control plasmids, then induced by PMA+EtOH (vehicle control), PMA+Dexamethasone (10 nM), PMA+Compound #53 (5 μM). Dual-Luciferase values were measured by Promega standard manual. Compound #53's GR transrepression activity on AP1 in AD293 cells is shown in FIG. 7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal structure | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com