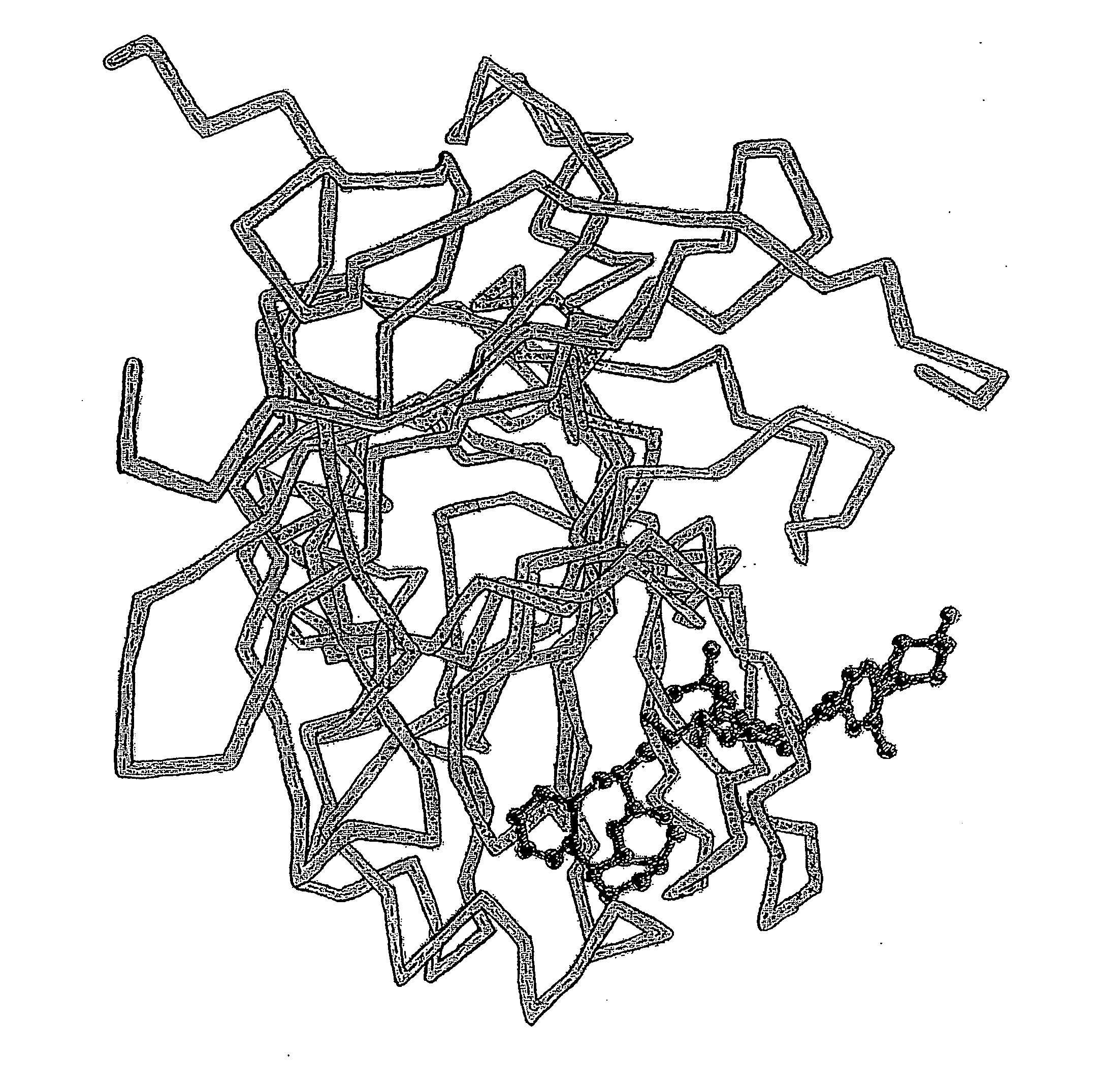
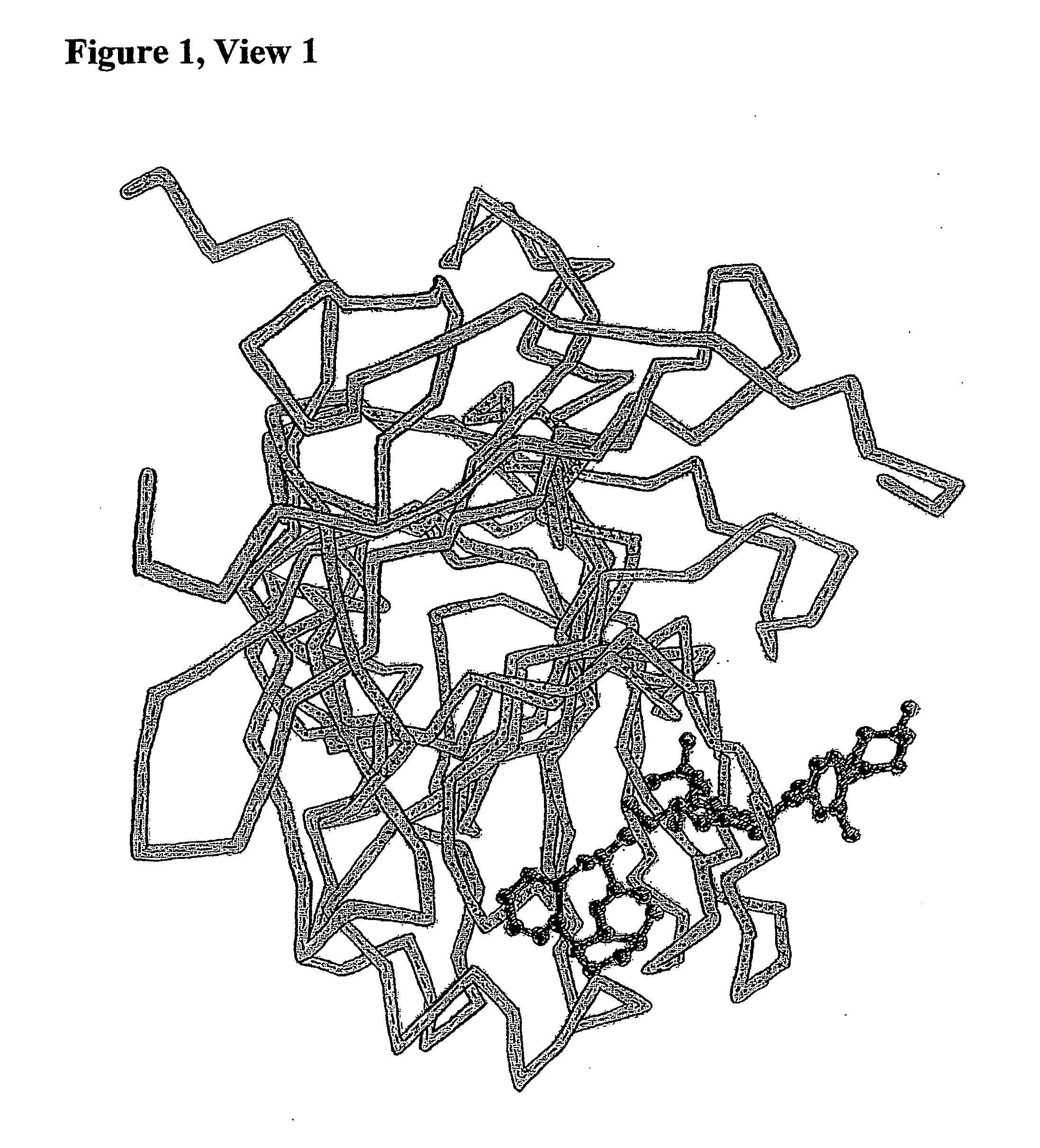
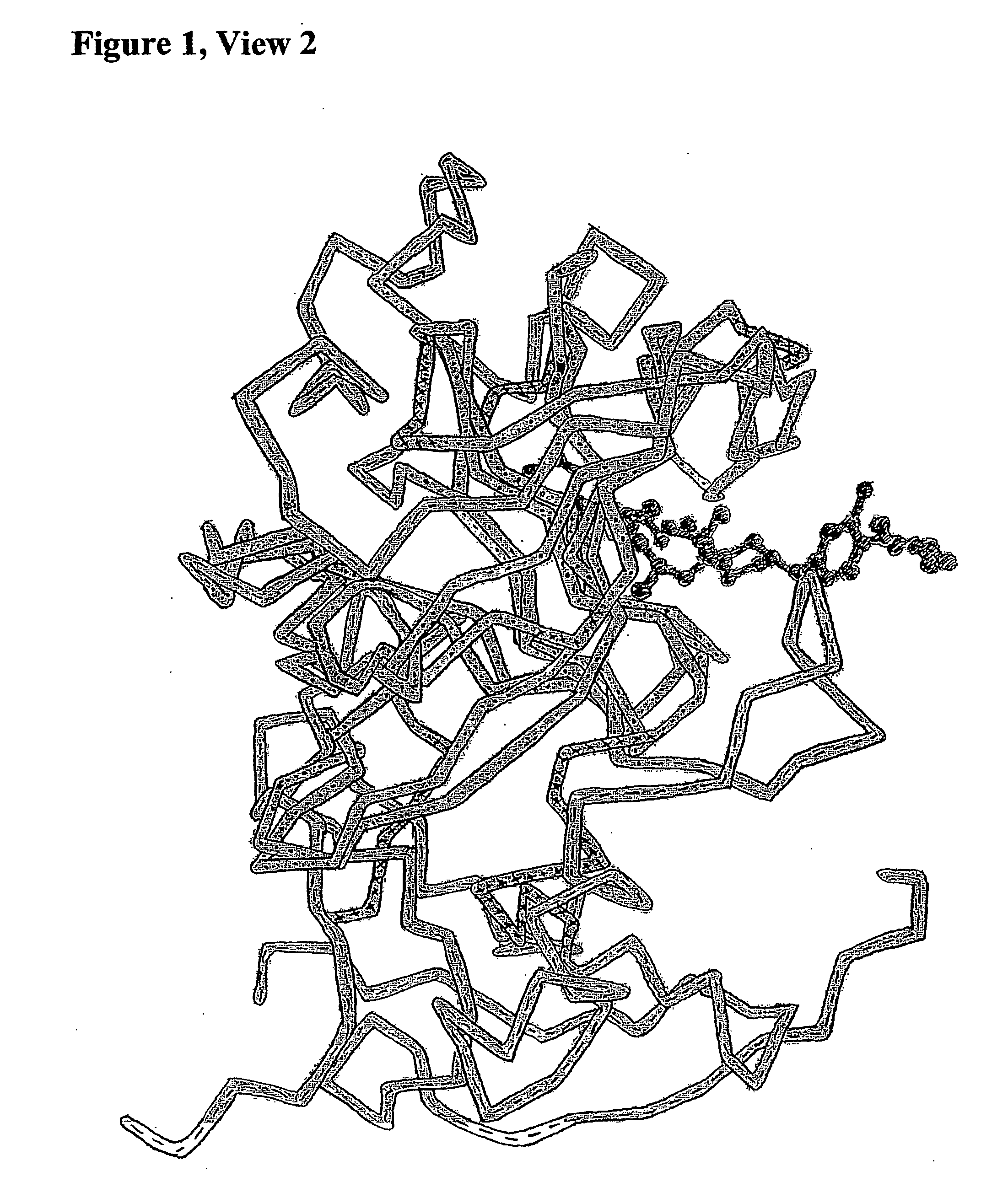
Methods for Treatment of HIV or Malaria Using Combinations of Chloroquine and Protease Inhibitors
a technology of protease inhibitor and chloroquine, which is applied in the field of drug combination, can solve the problems of difficulty per se in treating both aids and malaria, the most severe effects of these diseases, and the inability of several resource-poor countries to afford effective therapies, etc., and achieve the effect of restoring the sensitivity of drug-resistant isolates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0069]Infection Assays
[0070]Laboratory-adapted HIV-1.sub.IIIB and HIV-2.sub.CBL / 20 strans, the primary isolates HIV-1.sub.UG3 (Clade A, R5) and HIV-1.sub.VI 829 (Clade C, R5), both obtained from antiretroviral-naive subjects were used. These viruses are fully described in the paper by Savarino A. et al. referenced above. The HIV-1.sub.PAVIA12 isolate was also used. It was donated by Dr. Maurizia Debiaggi, University of Pavia, Italy, who also performed its genotypic analysis. It belongs to Clade B and was isolated from an individual with HAART failure. It possesses a genotypic profile of multi-drug resistance to all classes of antiretrovirals currently utilized in the medical practice (Mutations in the HIV-1 reverse transcriptase: 67N 69D 70R 74V 108I 181C 184V 219Q 228R; Mutations in the HIV-1 protease: 10I 20R 36I 46L 54V 55R 63P 71V 82A 90M). Viral stocks were titrated biologically by the 50% endpoint dilution method, using MT-2 cells (laboratory strains) or P...
example i
[0085]The purpose of this test was to analyze whether the addition of CQ to IDV might produce a level of HIV inhibition higher than that produced by IDV alone. HIV-1 IIIB-infected MT-4 cells were incubated in a medium containing 10 nM IDV in the presence or absence of increasing concentrations of CQ (1-6.25 •M). The IDV concentration chosen is close to the ECso in HIV-1 IIIB-infected MT-4 cells, as determined under our experimental conditions (data not shown). Addition of CQ did not result in significant differences in cell viability, thus excluding that the differences observed are due to a specific impairment of cell viability exerted by CQ (data not shown). The levels of p24 at 5 days post-infection in supernatants of cultures treated with CQ+IDV were lower than those in supernatants of cells treated with IDV alone (FIG. 3A; P<0.05, repeated-measures ANOVA). The effect of CQ was not dose-dependent, suggesting that the anti-HIV-1 potency of the CQ / IDV combination might decrease pa...
example ii
[0088]Before performing experiments on the CQ+PIs association, the effects of protease inhibitors when administered alone to P. falciparum parasited erythrocytes were evaluated. P. falciparum-parasited erythrocytes (starting parasitemia .congruent.1%) were cultivated for 48 h with concentrations of IDV and RTV lying within the range clinically observable in individuals treated with these PIs. Aliquots of the eryhthrocyte cell suspensions were then collected and assayed for P. falciparum LDH activity. Results indicated that RTV and IDV dose-dependently inhibited P. falciparum growth. (FIG. 4).
[0089]P. falciparum-parasited erythrocytes were cultivated for 48 h with decreasing concentrations of CQ in the presence or absence of a PI and then assayed for P. falciparum LDH activity as a measure of cell viability. FIGS. 5A and 5B show typical results obtainable with the combination of a PI with CQ. From these data it is evident that RTV and IDV restored the response to CQ in CQ-resistant P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com