Hydrogenation catalyst
a hydrogenation catalyst and catalyst technology, applied in the field of hydrogenation catalysts, can solve the problems of catalyst deactivation, change in catalyst structure, limiting useful life, etc., and achieve the effect of reducing the number of catalyst pellets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Comparison of Gamma-Alumina and Alpha-Alumina Supported Pd Catalysts for 1,1,1,2,3,3-hexafluoropropene hydrogenation
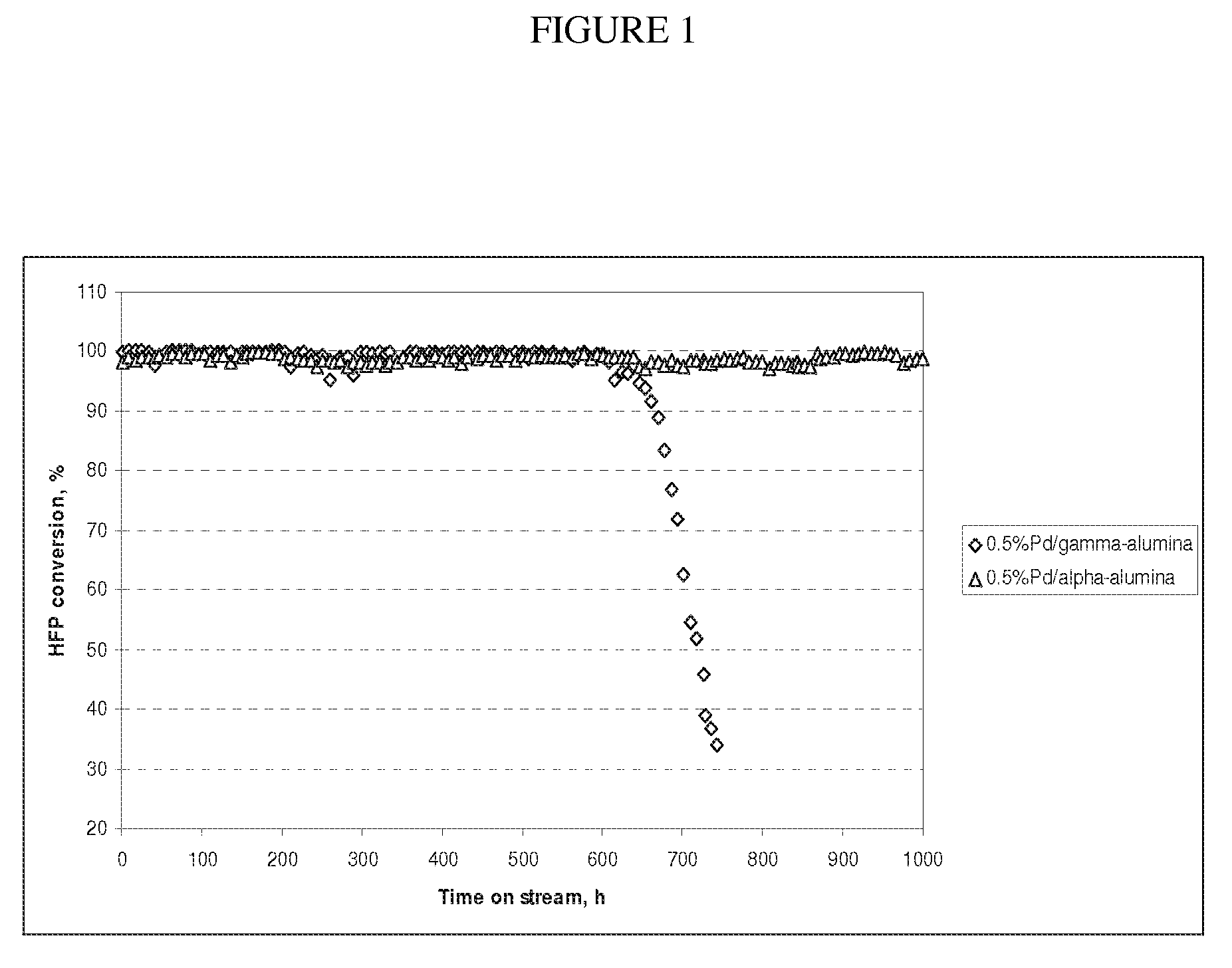
[0032]0.5 wt % Pd / gamma-alumina and 0.5 wt % Pd / alpha-alumina, which have a specific surface area of 243 and 33 m2 / g, respectively, were compared for 1,1,1,2,3,3-hexafluoropropene (HFP) hydrogenation. About 2 g of catalyst diluted with 20 ml of Monel packing was charged into a ¾″ Monel tube reactor and was in-situ reduced in 10% H2 / N2 flow for 2 hours at 200° C. HFP was fed into reactor at a rate of 5 g / h, and H2 was co-fed according to a mole ratio of H2 / HFP equal to 1.5. As shown in Table 2, both catalysts initially provided a near complete HFP conversion and a 236ea selectivity of above 99.5%. Nevertheless, as shown in FIG. 1, while no deactivation was noted over the 0.5 wt % Pd / alpha-alumina catalyst even after 1000 h on stream, rapid deactivation was observed over the 0.5 wt % Pd / gamma-alumina beginning around 600 h on stream. This indicates that the alpha-alumina...
example 2
Hydrogenation of 1,1,1,2,3,3-hexafluoropropene Over Alpha-Alumina Supported Pd Catalyst
[0033]0.5 wt % Pd / alpha-alumina catalyst, which has a specific surface area of 33 m2 / g, was used for 1,1,1,2,3,3-hexafluoropropene (HFP) hydrogenation. About 1 g of catalyst diluted with 10 ml of Monel packing was charged into a ¾″ Monel tube reactor and was in-situ reduced in 10% H2 / N2 flow for 2 hours at 200° C. HFP was fed into reactor at a rate of 65 g / h, and H2 was co-fed according to a mole ratio of H2 / HFP equal to 1.5. GC analysis of the product stream showed that the catalyst provided an HFP conversion of around 55% and a 245eb selectivity of about 99.5%. No deactivation was noted during the period of time of the test which lasted for 800 hours, indicating the alpha-alumina supported Pd catalyst can provide stable activity for HFP hydrogenation.
example 3
Hydrogenation of 1,1,1,2,3-pentafluoropropene Over Alpha-Alumina Supported Pd Catalyst
[0034]0.5 wt % Pd / alpha-alumina catalyst, which has a specific surface area of 33 m2 / g, was used for 1,1,1,2,3-pentafluoropropene (1225ye) hydrogenation. About 0.5 g of catalyst diluted with 10 ml of Monel packing was charged into a ¾″ Monel tube reactor and was in-situ reduced in 10% H2 / N2 flow for 2 hours at 200° C. 1225ye was fed into reactor at a rate of 30 g / h, and H2 was co-fed according to a mole ratio of H2 / 1225ye equal to 1.5. GC analysis of the product stream showed that the catalyst provided a 1225ye conversion of around 45% and a 245eb selectivity of about 98.5%. No deactivation was noted during the period of time of the test which lasted for 800 hours, indicating the alpha-alumina supported Pd catalyst can provide stable activity for 1225ye hydrogenation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com