Long lasting fusion peptide inhibitors for HIV infection
a fusion peptide, long-lasting technology, applied in the direction of peptide/protein ingredients, peptide sources, drug compositions, etc., can solve the problems of short half-life in vivo of c34, reducing the effective anti-viral activity,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
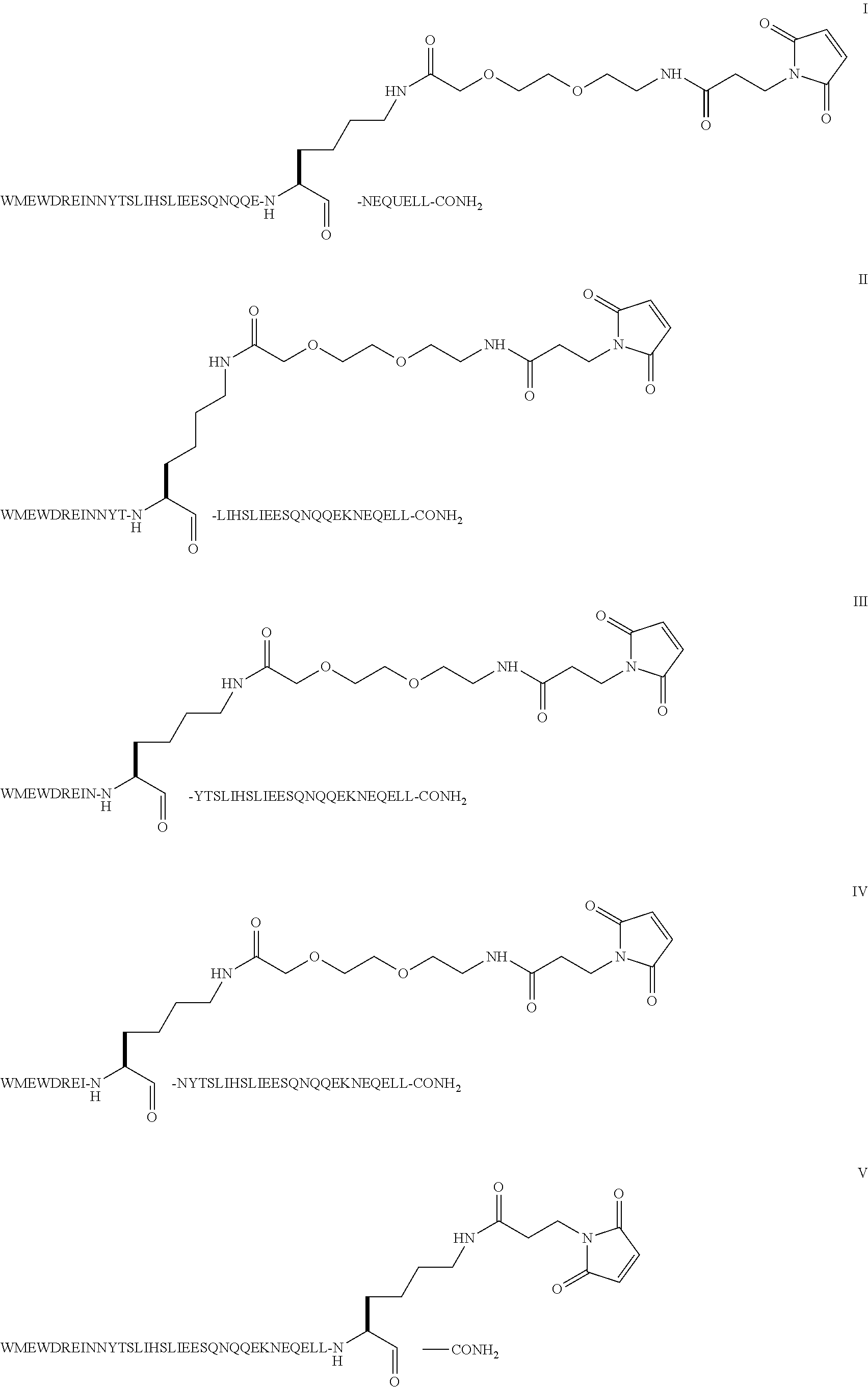
Compound of Formula I
[0047]Step 1: The example describes the solid phase peptide synthesis of the compound on a 100 μmole scale. The following protected amino acids were sequentially added to resin: Fmoc-Leu-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Lys(Aloc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Glu(tBu)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Met-OH, Fmoc-Trp(Boc)-OH. They were dissolved in N,N-dimethylformamide (DMF) and, according to the sequence, activated using O-benzotriazol-1-yl-N,N,N′,N′-tetramethyl-uronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA). Removal of the Fmoc protecting group was achieved using a solution of 20% (V / ...
example 2
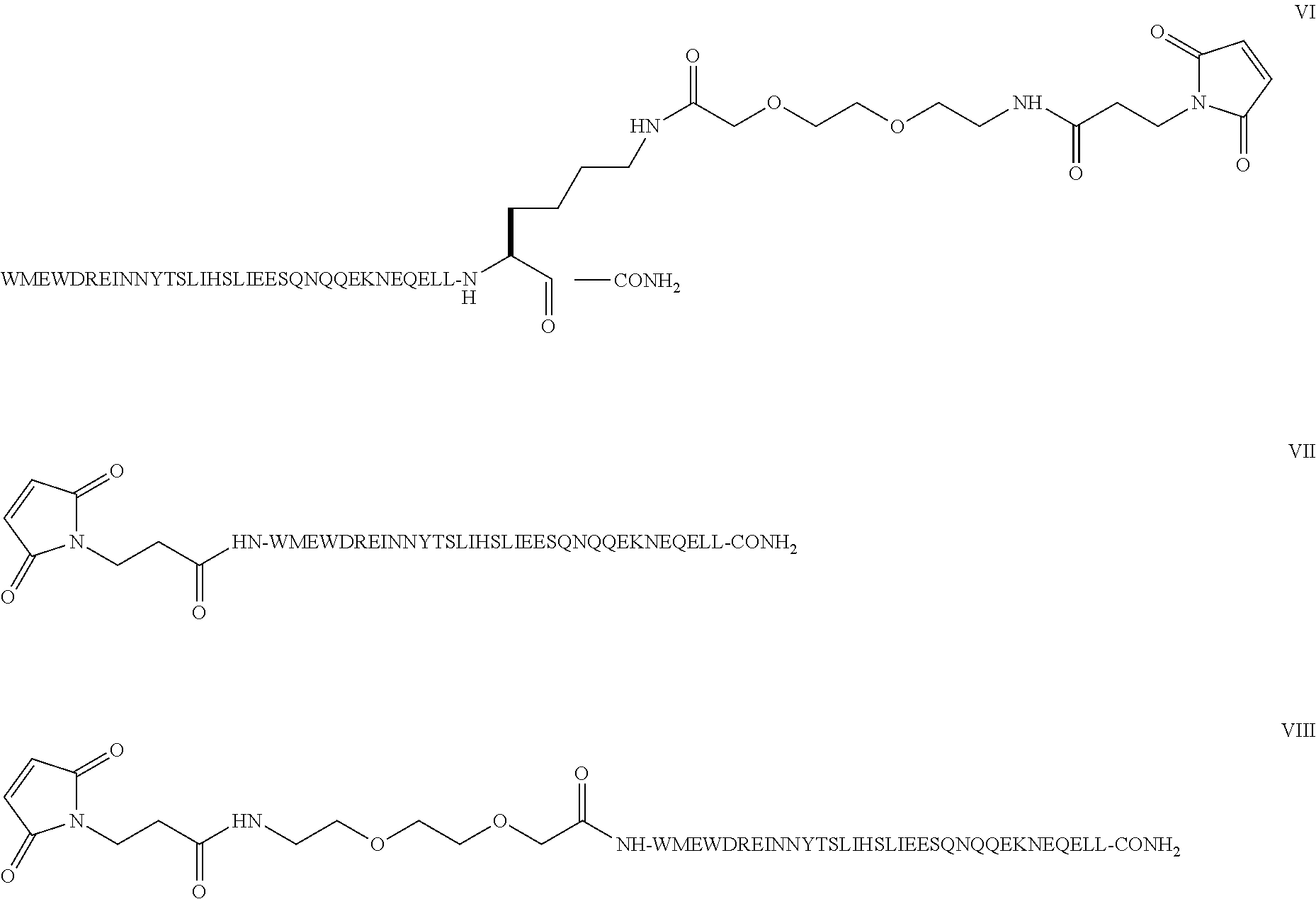
Compound of Formula II
[0048]Step 1: The example describes the solid phase peptide synthesis of the compound on a 100 mmole scale. The following protected amino acids were sequentially added to resin: Fmoc-Leu-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Lys(Aloc)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Glu(tBu)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Met-OH, Fmoc-Trp(Boc)-OH. They were dissolved in N,N-dimethylformamide (DMF) and, according to the sequence, activated using O-benzotriazol-1-yl-N,N, N′,N′-tetramethyl-uronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA). Removal of the Fmoc protecting group was achieved using a solution of 20% (...
example 3
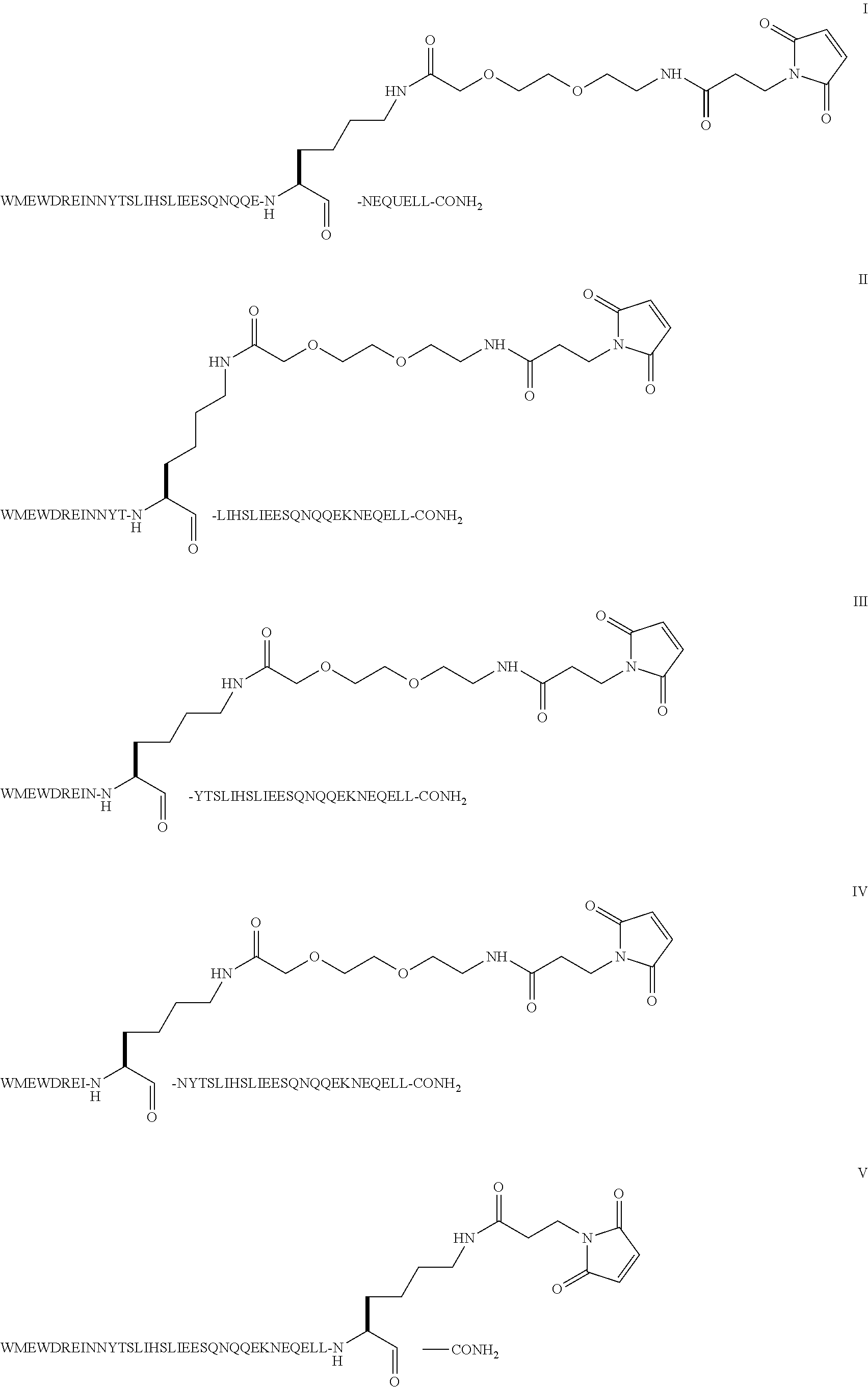
Compound of Formula III
[0049]Step 1: The example describes the solid phase peptide synthesis of the compound on a 100 μmole scale. The following protected amino acids were sequentially added to resin: Fmoc-Leu-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Lys(Aloc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Glu(tBu)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Met-OH, Fmoc-Trp(Boc)-OH. They were dissolved in N,N-dimethylformamide (DMF) and, according to the sequence, activated using O-benzotriazol-1-yl-N,N,N′,N′-tetramethyl-uronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA). Removal of the Fmoc protecting group was achieved using a solution of 20% (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com