Method for distribution of a drug
a technology for drugs and distribution methods, applied in the field of methods and systems for drug distribution, can solve the problems of defective patient and prescriber forms, computer will not generate authorization forms, and cannot allow passage to the next field or phase of completion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Controlled Distribution of TYSABRI® for Relapsing MS
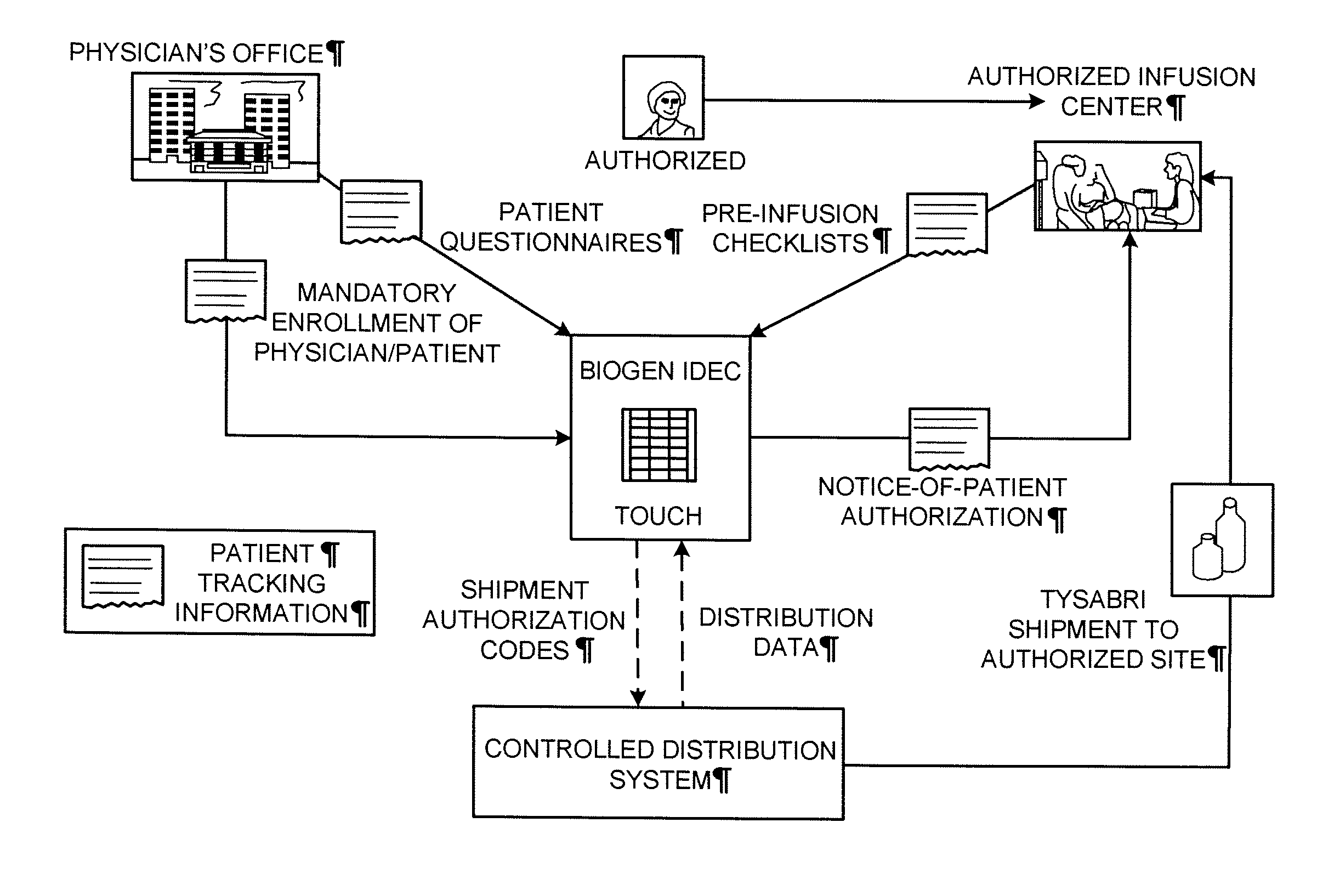
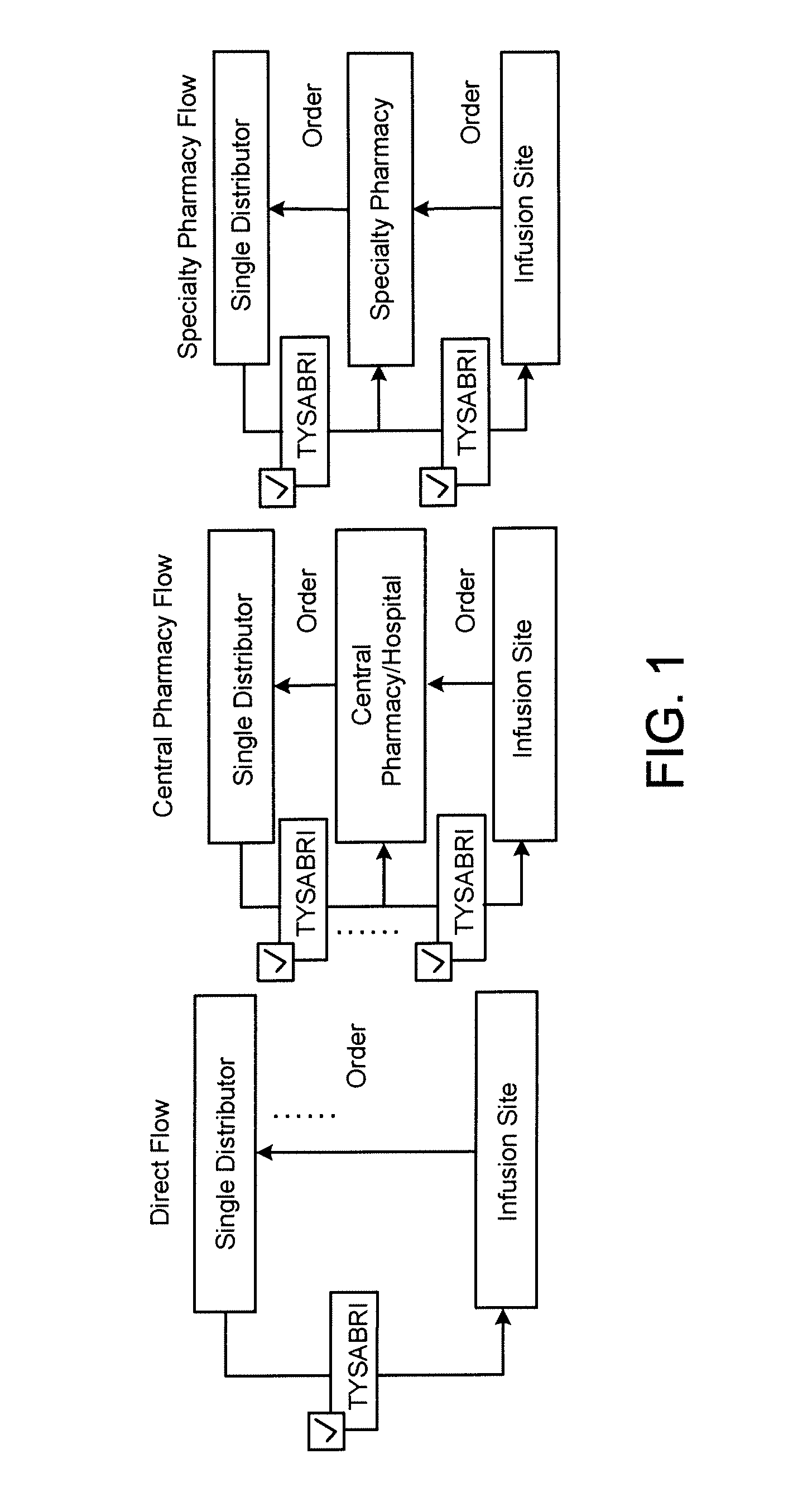
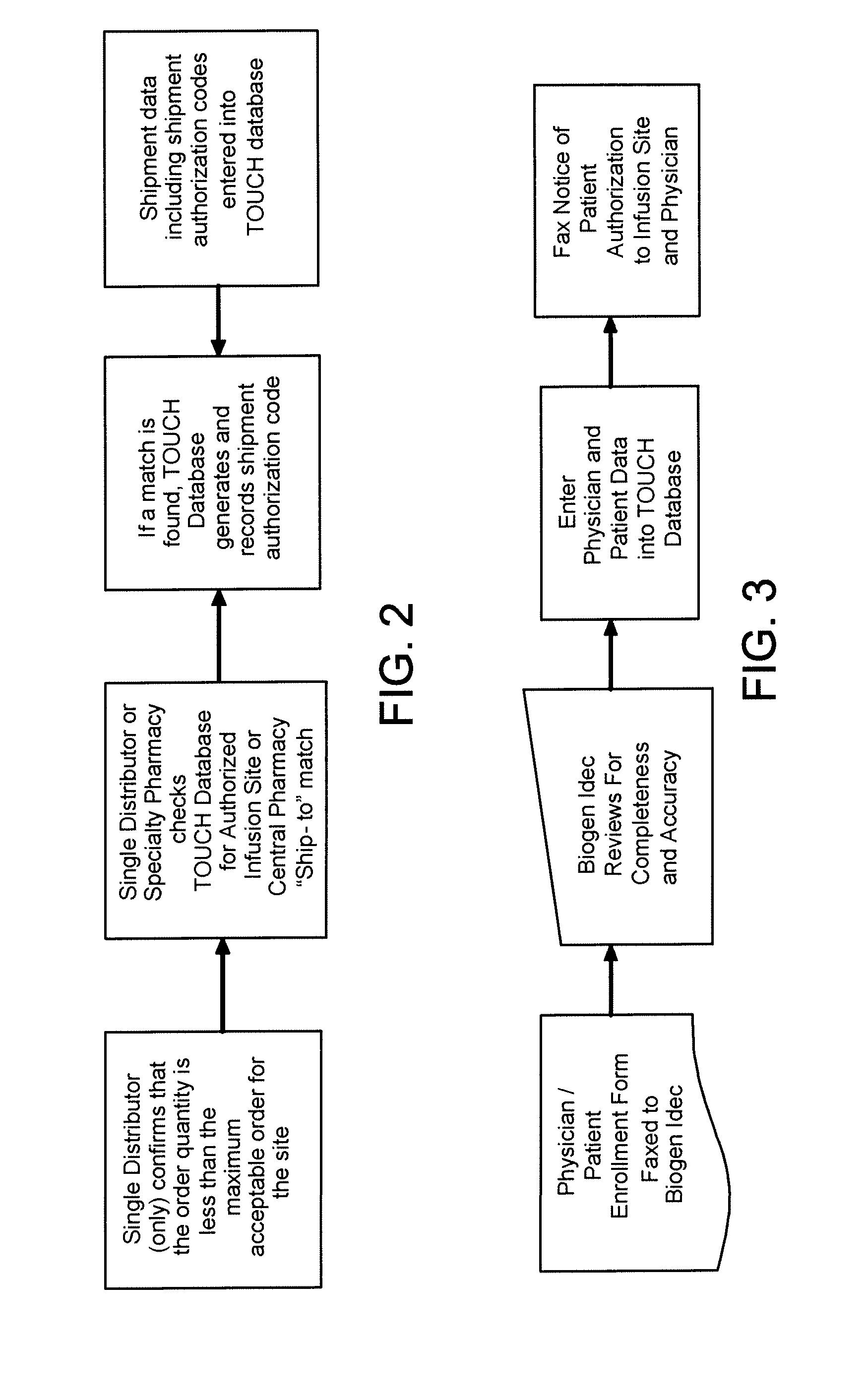
[0179]The following example describes a plan for the controlled distribution of TYSABRI® by Biogen Idec. Although the plan refers to Biogen Idec as the central administrator, it is applicable to any central administrator. Although the plan refers to TYSABRI®, the plan can be adapted to other drugs. Although the following plan includes many features, a plan may include all or a subset of these features as needed for a particular application.
1. Risk Management Plan Background
1.1 Overview
[0180]Biogen Idec (the Sponsor) has developed a comprehensive risk management plan (RiskMAP), consisting of both risk assessment and a risk minimization features. This document outlines the goals, objectives, and processes of the revised TYSABRI® RiskMAP based on discussions with the Agency and the recommendations of the Peripheral and Central Nervous System Drugs Advisory Committee (Advisory Committee). The TYSABRI® RiskMAP is designed to promote inf...
example 2
Exemplary Computer Implementation
[0488]In an exemplary computer implementation, FIG. 9 is a block diagram of computing devices and systems 400, 450. Computing device 400 is intended to represent various forms of digital computers, such as laptops, desktops, workstations, personal digital assistants, servers, blade servers, mainframes, and other appropriate computers. Computing device 450 is intended to represent various forms of mobile devices, such as personal digital assistants, cellular telephones, smartphones, and other similar computing devices. The components shown here, their connections and relationships, and their functions, are meant to be exemplary only, and are not meant to limit implementations of the inventions described and / or claimed in this document.
[0489]Computing device 400 includes a processor 402, memory 404, a storage device 406, a high-speed interface 408 connecting to memory 404 and high-speed expansion ports 410, and a low speed interface 412 connecting to l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com