Method for enhancing immune responses in mammals
a technology of immune response and enhancing antibody, applied in the field of mammals' immune response enhancement, can solve the problems of reducing the vigor of immune response, altering immune responsiveness, and reducing the production of immune system stimulators in mammals, so as to reduce the abundance of immune stimulators and minimize toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production, Purification, and Characterization of the Immune System Inhibitor, Human sTNFRI
[0052]The sTNFRI used in the present studies was produced recombinantly in cell culture. The construction of the eukaryotic expression plasmid, the methods for transforming cultured cells, and for assaying the production of sTNFRI by the transformed cells have been described (Selinsky, et al., supra). The sTNFRI expression plasmid was introduced into HeLa cells (American Type Culture Collection #CCL 2), and an sTNFRI-producing transfectant cell line was isolated by limiting dilution. This cloned cell line was cultured in a fluidized-bed reactor at 37.degree. C. in RPMI-1640, supplemented with 2.5% (v / v) fetal bovine serum and penicillin / streptomycin, each at 100 micrograms per milliliter. sTNFRI secreted into the culture medium was purified by affinity chromatography on a TNF-Sepharose-4B affinity matrix essentially as described (Engelmann, et al., J. Biol. Chem. 265:1531-1536).
[0053]sTNFRI wa...
example 2
Production of Absorbent Matrices
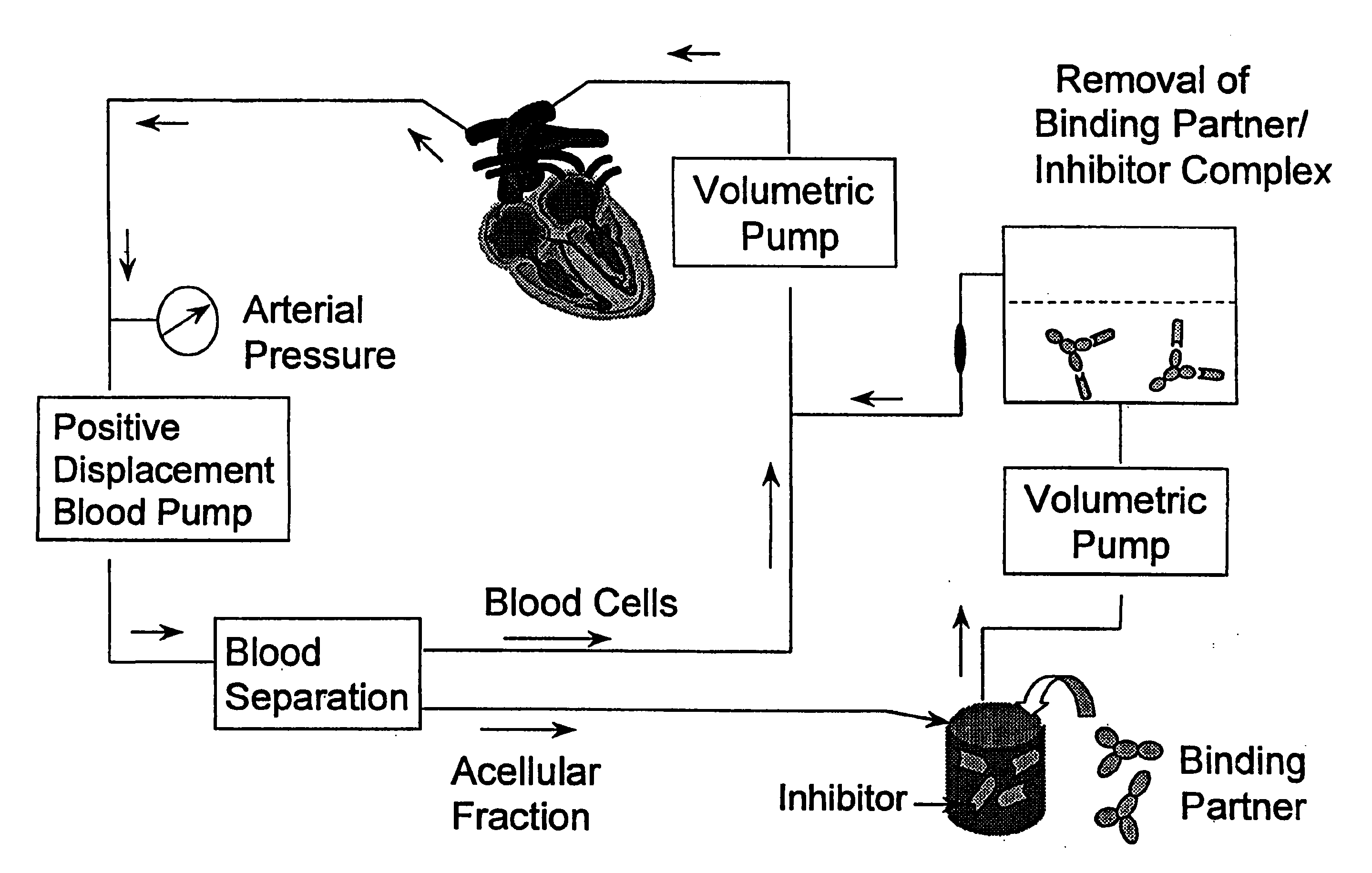
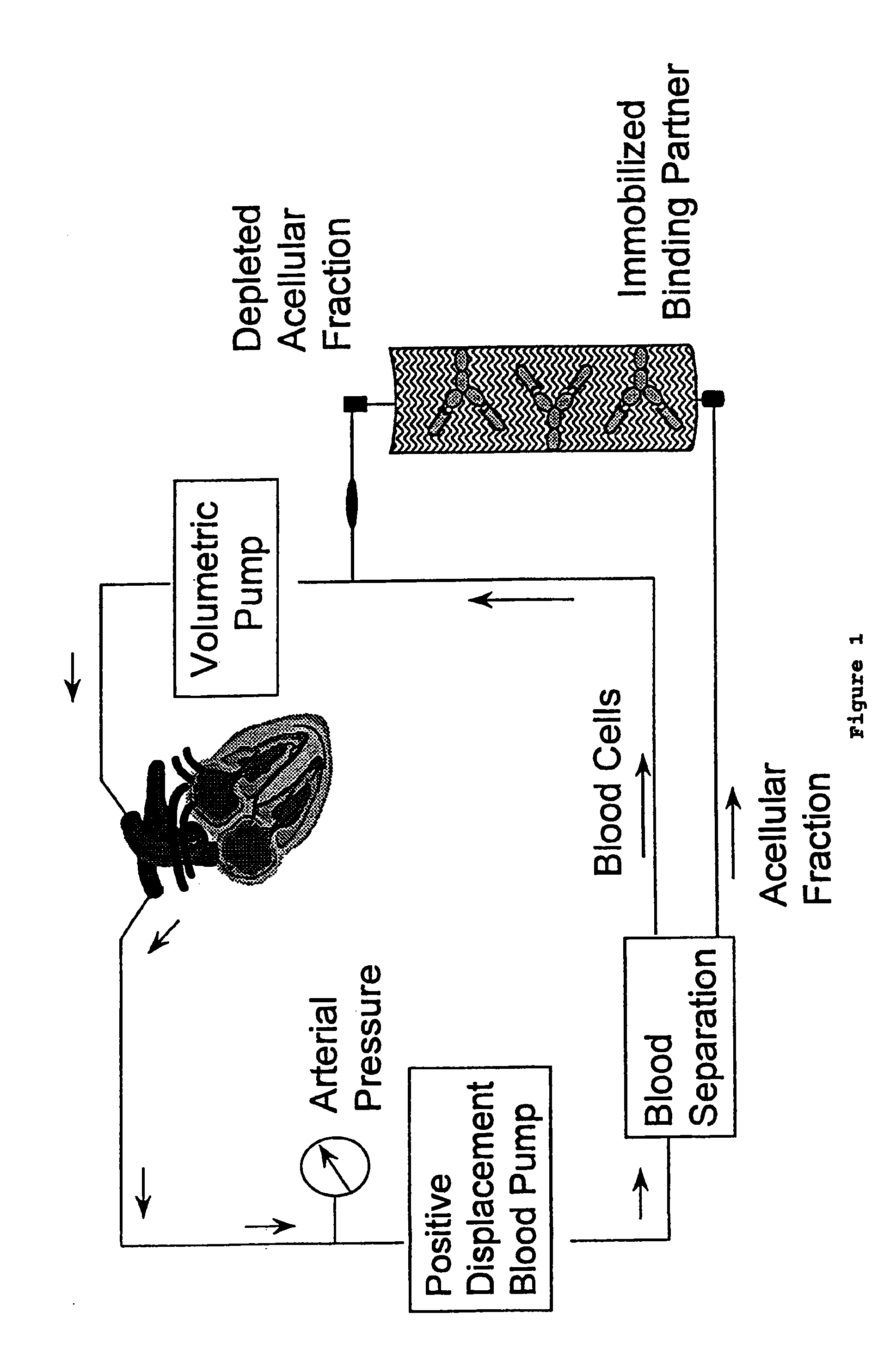
[0054]Binding partners used in the present studies include an IgG fraction of goat anti-human sTNFRI antisera (R&D Systems, Cat. #AF-425-PB) and a monoclonal antibody reactive with sTNFRI (Biosource International, Cat. #AHR3912). An additional monoclonal antibody, OT145 (Cat. #TCR1657), reactive with a human T cell receptor protein, was purchased from T Cell Diagnostics (now, Endogen) and was used as a control binding partner. Each of these respective binding partners was covalently conjugated to cyanogen bromide-activated Sepharose-4B (Pharmacia Biotech), a macroporous bead which facilitates the covalent attachment of proteins. Antibodies were conjugated at 1.0 milligram of protein per milliliter of swollen gel, and the matrices were washed extensively according to the manufacturer's specifications. Matrices were equilibrated in phosphate buffered saline prior to use.
example 3
Depletion of the Immune System Inhibitor, sTNFRI, from Human Plasma Using Absorbent Matrices
[0055]Normal human plasma was spiked with purified sTNFRI to a final concentration of 10 nanograms per milliliter, a concentration comparable to those found in the circulation of cancer patients (Gadducci, et al., supra). One milliliter of the spiked plasma was mixed with 0.25 milliliter of the respective absorbent matrices at 0.degree. C. and a plasma sample was removed at time=0. The samples were warmed rapidly to 37.degree. C., and incubated with agitation for an additional 45 minutes. Plasma samples were removed for analysis at 15 minute intervals and, immediately after collection, were separated from the beads by centrifugation. Samples were analyzed by ELISA to quantify the levels of sTNFRI, and to permit the determination of the extent of depletion.
[0056]FIG. 3 shows the results of the sTNFRI depletion. The absorbent matrix produced with the goat anti-human sTNFRI polyclonal antibody r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com