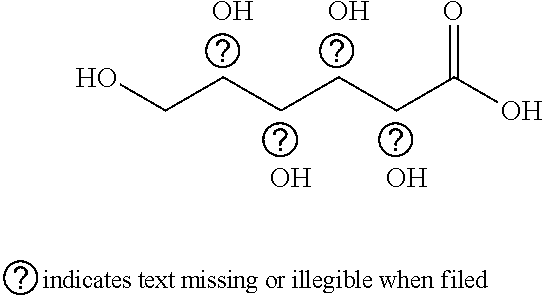
Acid cleaning and corrosion inhibiting compositions comprising gluconic acid
a technology of gluconic acid and composition, which is applied in the field of acid cleaners, can solve the problems of limited usefulness, corrosion, and still corrode, and achieve the effects of less expensive, less corrosion and cleaning, and greater gluconic acid conten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Metal Corrosion Test
[0098]The following test method describes an accepted, but not exclusive, procedure for metal corrosion testing as outlined in the American Society for Testing and Materials (ASTM), Volume 3.02, G31-72 and 3.02, G1-90, and as outlined in the Klenzade Good Laboratory Practice standard test method, K004-01-01, which is in compliance with Environmental Protection Agency (EPA) registration and UN / DOT for corrosion testing. This test method was employed in Examples 2 and 3.
[0099]Metal strips are pre-cleaned, weighed, and put into glass bottles with product solution (100% concentration for UN / DOT) and placed at appropriated temperature (130° F. (54.5° C.) for UN / DOT). After the specified time, the corroded metal strips are then cleaned, weighed, and weight loss is determined. Corrosion rates are directly proportional to the mass loss of the metal strip and inversely proportional to the strip area, density, and time of exposure to the test solution. A corrosion rate exc...
example 2
Project: Urea Sulfate Corrosion
Begin Urea Sulfate+Gluconic Acid Corrosion Test
[0134]1 L of an acid cleaning solution of urea sulfate+gluconic acid solution was made for testing corrosion
weight%715.471.54Water166.616.66H2SO4 93%115.011.50Urea Prill3.00.30Gluconic acid 50%1000.0100.00[0135]One coupon each of Ni, 304SS, 316SS, 410SS were tested. Each coupon was placed in an 8 oz glass bottle and submerged with ˜200 mL concentrated urea sulfate+gluconic acid solution. The bottles were then placed in a preheated water bath at set at 160° F.[0136]Next one coupon each of Ni, 304SS, 316SS, 410SS were tested by placing 2 drops concentrated urea sulfate+gluconic acid solution on top of coupon near the middle and the coupons were let sit at room temp. No obvious initial reaction between solution and any of the coupons was observed.[0137]The 410SS and 304SS coupons were removed from water bath after one hour and 15 minutes because both failed. The solutions were green with lots of bubbles formi...
example 3
Urea Sulfate+Gluconic Acid Corrosion Test Preparation
[0142]Coupons were weighed and measured coupons for compatibility testing per the table below.
NisurfaceInitialdeptharea (SA)weightcouponlength (in)width (in)(in)(in{circumflex over ( )}2)(g)100%13.0061.0000.0636.51727.1555solution23.0151.0030.0626.54627.133233.0041.0010.0626.51126.8191Initialdepthweightcouponlength (in)width (in)(in)SA (in{circumflex over ( )}2)(g)316SS100%13.0030.9970.0606.46823.1391solution23.0030.9980.0606.47423.183633.0020.9960.0606.46023.1484304SS100%13.0020.9990.0586.46222.2192solution23.0110.9980.0586.47522.245433.0100.9990.0586.47922.2686410SS100%693.0061.0040.0506.43719.0677solution703.0061.0050.0506.44319.0878713.0041.0060.0506.44519.0685
Urea Sulfate+Gluconic Acid Room Temp Corrosion Test:
[0143]24 L of urea sulfate+gluconic acid solution was prepared for corrosion testing.
1717.071.54water399.816.66H2SO4 93%276.011.50prilled urea7.20.30gluconic acid 50%2400.0100.00
˜200 mL was poured in each 8 oz. glass bo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com