Use of an ang-(1-7) receptor agonist in acute lung injury
a technology of ang-(1-7) receptor and agonist, which is applied in the direction of antibacterial agents, peptide/protein ingredients, extracellular fluid disorder, etc., can solve the problem that no therapeutic pharmacological intervention could so far improve the clinical outcome of ali/ards, and achieve the effect of conserving or improving the agonistic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
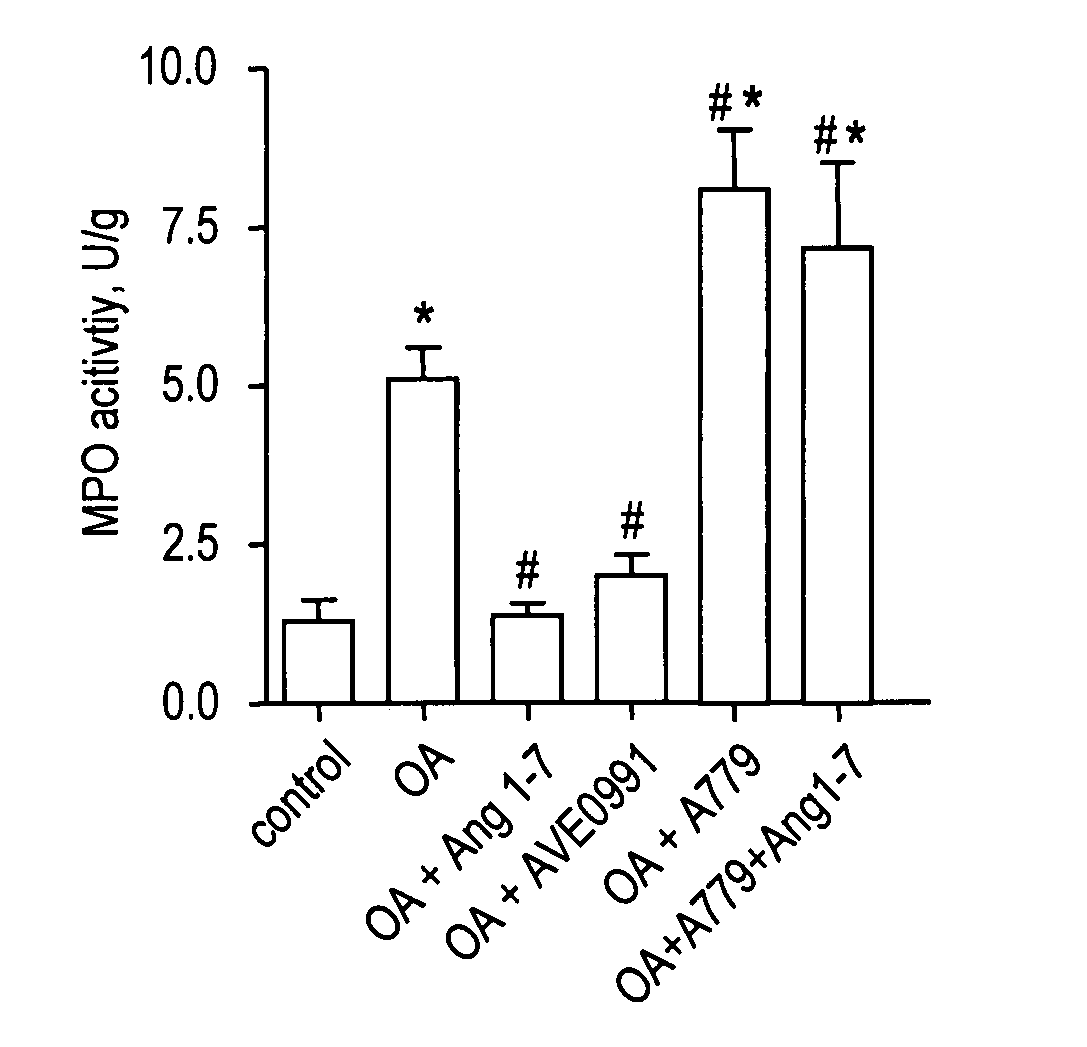
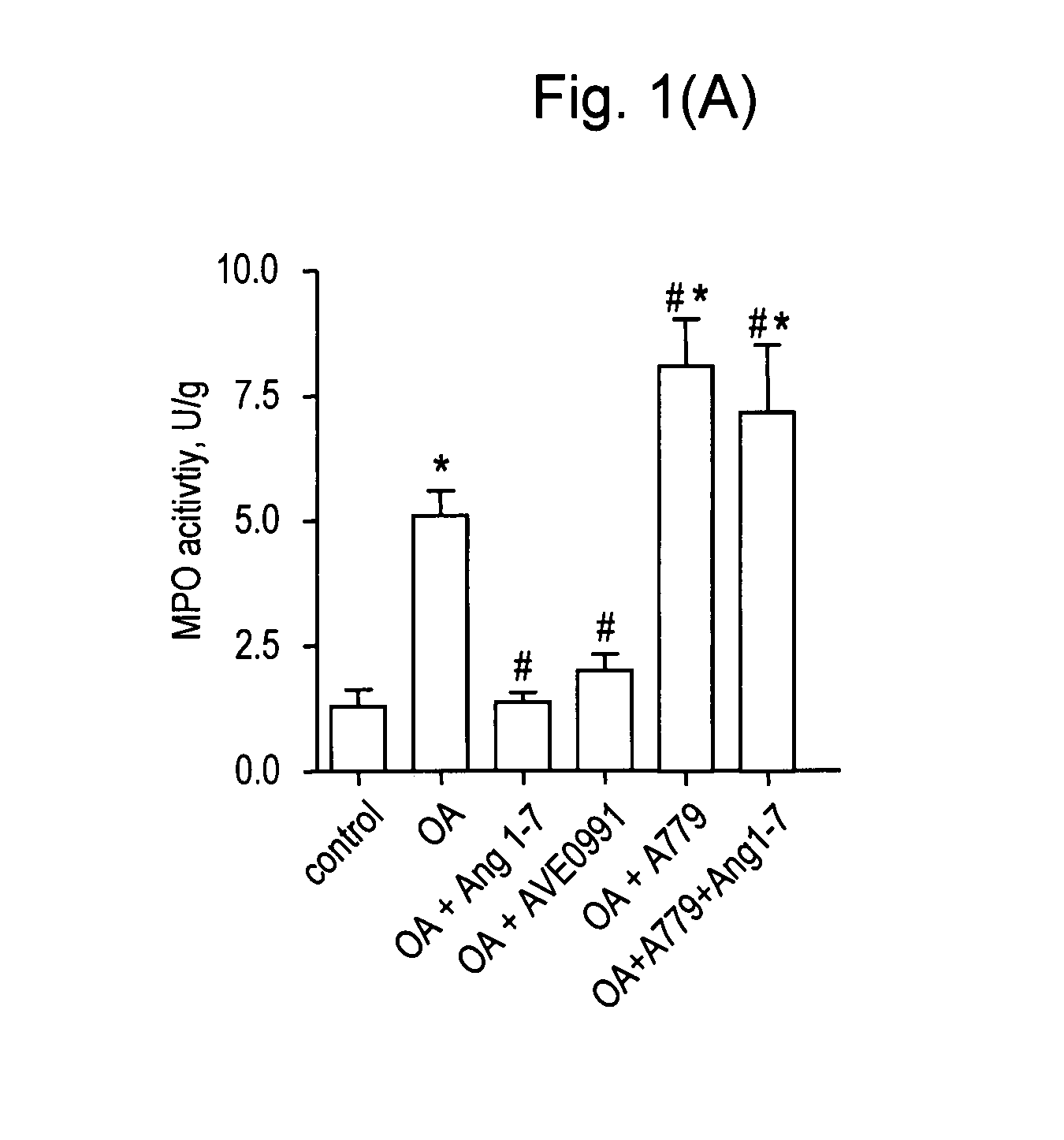
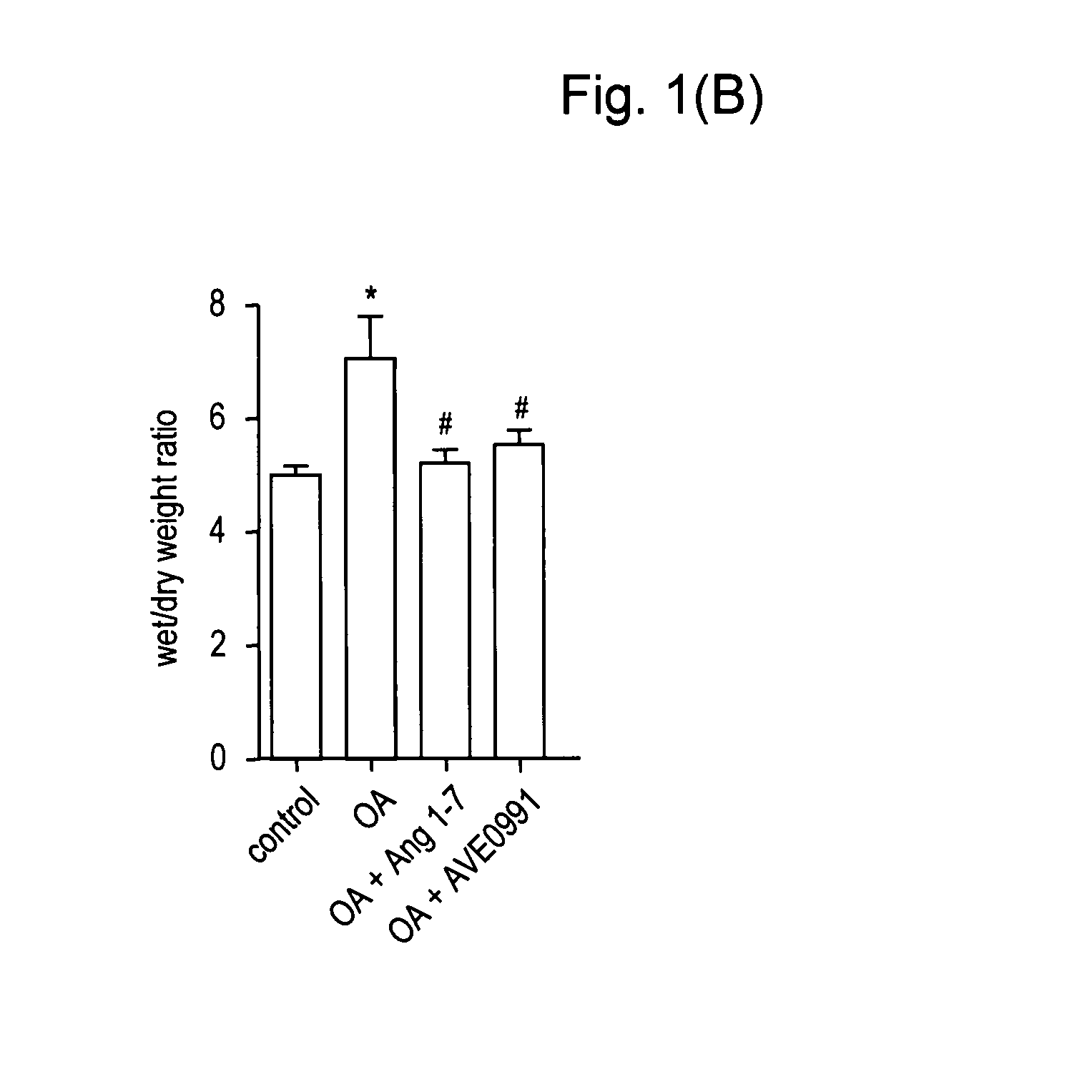
[0063]Animals. Experiments were performed in male Sprague-Dawley rats (Charles River Wiga GmbH, Sulzfeld, Germany) with a body weight (bw) of 330-360 g. Animals received care in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 7th edition 1996). The study was approved by the local animal care and use committee.
[0064]Surgical preparation and hemodynamic monitoring. Rats were anesthetized by intraperitoneal injection of medetomidine (0.5 mg / kg bw, Domitor®, Dr. E. Graeub A G, Basel, Switzerland), fentanyl (0.05 mg / kg bw, JanssenCilag, Neuss, Germany) and midazolam (5 mg / kg bw, Dormicum®, Roche, Basel, Switzerland) as previously described (17). Following tracheotomy, the trachea was cannulated and ventilation was established (Advanced Animal Respirator, TSE Systems GmbH, Bad Homburg, Germany) with a tidal volume of 6 ml / kg bw at 80 breaths / min. Catheters (internal diameter 0.58 mm; Sims Portex Ltd., Hythe, United Kingdom) w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| lung permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com