Compositions and methods for treating or preventing atrial fibrillation
a technology of atrial fibrillation and compositions, applied in the direction of drug compositions, cardiovascular disorders, biocide, etc., can solve the problems of irregular heart rate, irregular heart rate, and af is a major risk factor for stroke and other embolic events, so as to prevent atrial fibrillation and prevent atrial fibrillation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0054]Canine CHF Model. Sterile surgery for pacemaker implantation was performed in canine subjects. The pacemaker was programmed to pace the right ventricle for 3 weeks at a rate of 240 beats per minute. CHF was confirmed by left ventricular dysfunction assessed by serial echocardiogram and by clinical assessment (e.g. ascites, tachypnea, reduced physical activity).
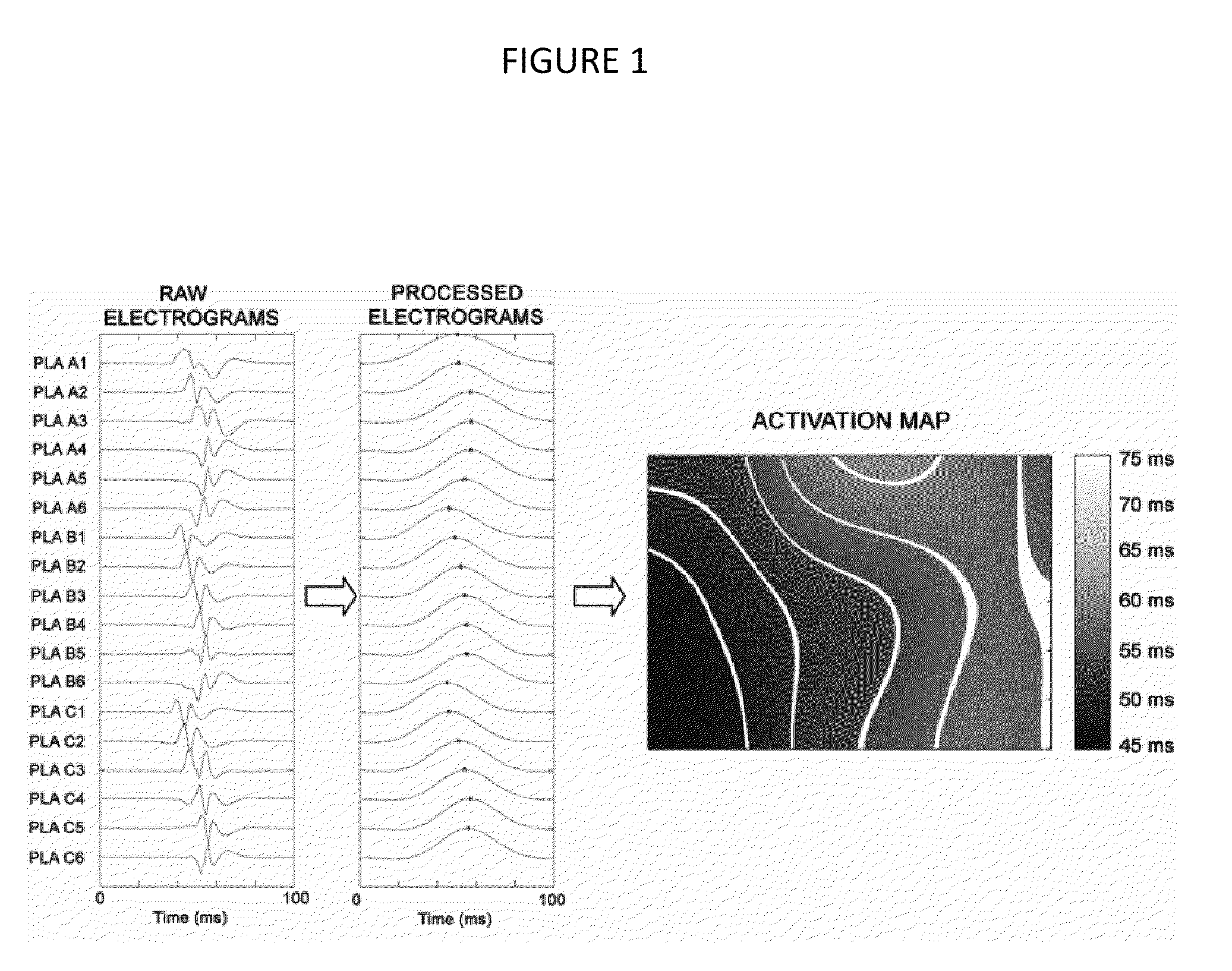
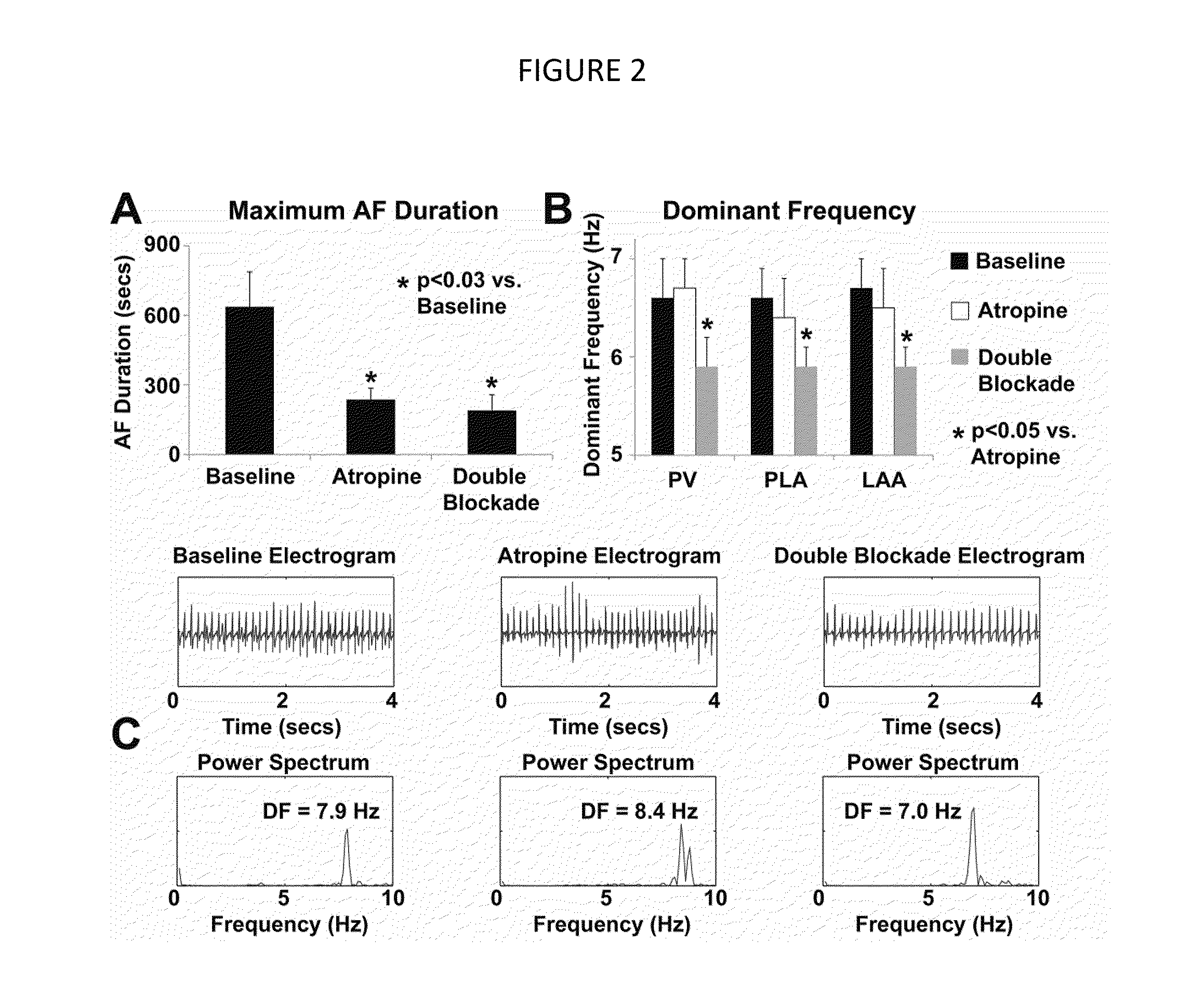
[0055]Experimental Setup. After the 3 weeks of pacing, the subjects were intubated and a median sternonomy was performed under general anesthesia with isoflurane. High density plaques were applied to the left inferior pulmonary vein (PV; 8×5 electrodes; 2.5 mm spacing), the posterior left atrium (PLA; 7×3 electrodes, 5 mm spacing) and the left atrial appendage (LAA; 7×3 electrodes, 5 mm spacing) for bipolar electrogram recordings and pacing. The PV plaque was placed circumferentially around the vein while the other two plaques were laid flat on the PLA and LAA epicardium. The left cervical vagus nerve was isolated and at...
example 2
[0070]Canines were subjected to heart failure (HF) by rapid ventricular pacing (240 / min for 3 weeks). Vagal stimulation induced ERP shortening (VS-ΔERP)(ms) was assessed in the test canines as well as controls at multiple sites in the LA. VS-ΔERP was reassessed in the presence of an acetylcholineesterase (AChE) inhibitor (physostigmine). Explanted LA were subjected to 1) ELISA for acetylcholinesterase (AChE) activity, 2) AChE immunostaining and 3) radioactive M2 receptor binding assay.
[0071]VS-ΔERP was significantly reduced in HF, but was restored by physostigmine (from 10±4 to 28±4). The number of parasympathetic ganglia in the LA was increased in HF (HF vs. control=3.8±3 cm−2 vs. 0.95±1.5 cm−2). AChE activity was also greater in HF. There was no change in M2 binding activity in HF compared to control. There was a profound increase in parasympathetic innervation, and a decrease in parasympathetic responsiveness in the HF LA, despite intact M2 receptor binding. Experiments conducted...
example 3
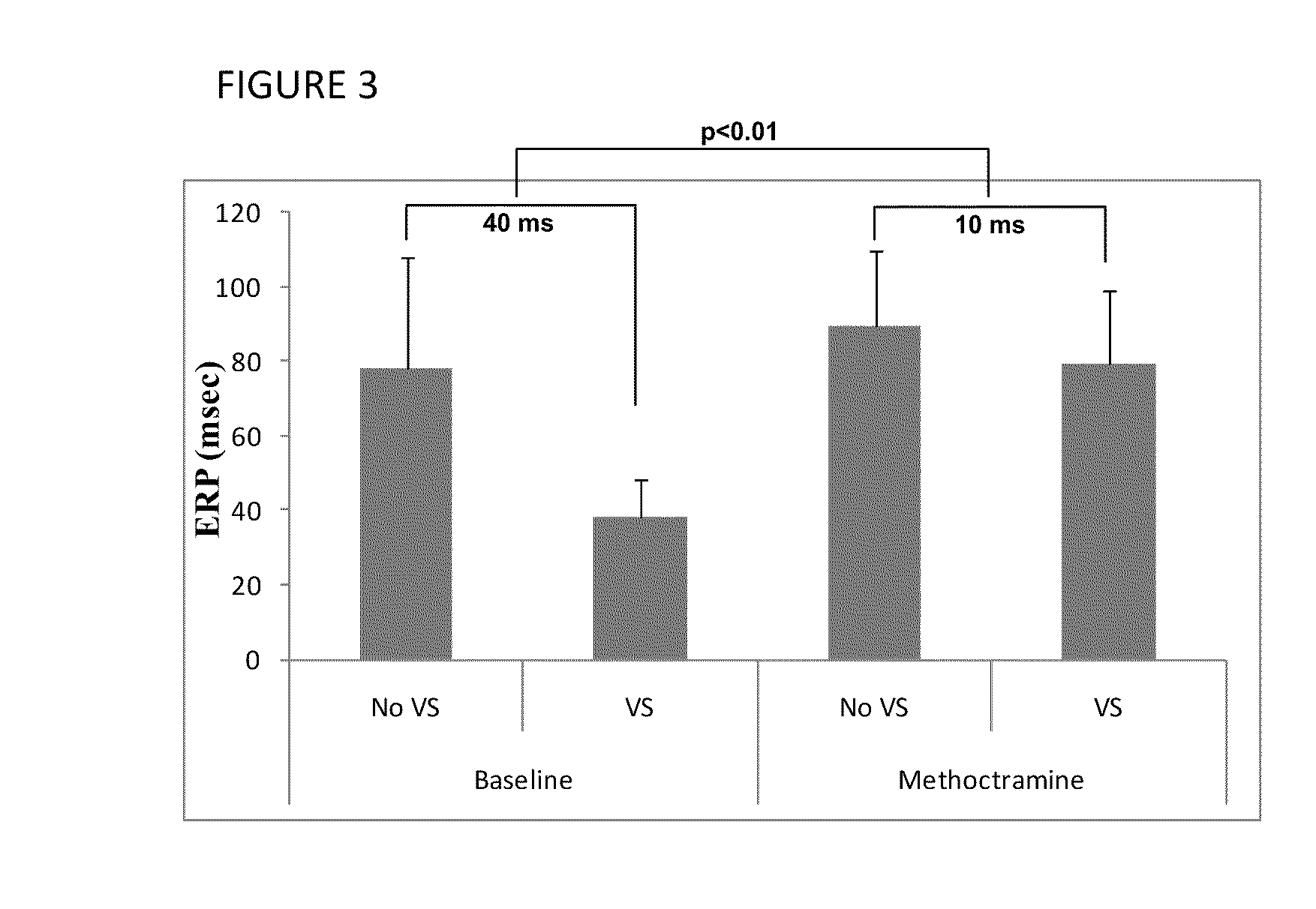
[0072]Canine Arrhythmia Model. Methoctramine, a muscarinic receptor blocker that is selective for blocking the M2 receptor subtype (Tumiatti, V. et al. Bioorg. Med. Chem. 2007, 15, 2312-2321), was evaluated for its ability to decrease effective refractory period and atrial fibrillation induced by vagal stimulation. Open chest electrophysiological mapping was performed in a canine model as previously described (Arora, R. et al. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H134-H144). Recording plaques were placed on the posterior left atrium (PLA), left superior pulmonary vein (PV) and the left atrial appendage (LAA). At baseline, effective refractory periods (ERPs) were obtained at multiple sites on each plaque in the absence and presence of vagal stimulation (VS). AF inducibility was also assessed under each of these conditions i.e. with and without VS. After baseline measurements had been made, methoctramine tetrahydrochloride hydrate (20 microgram / kg), dissolved in normal sali...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Selectivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com