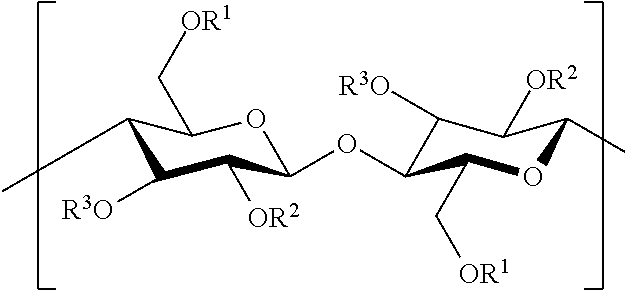
Cellulose ester compositions
a technology of cellulose esters and compositions, applied in the field of cellulose ester chemistry, can solve the problems of difficult dispersibility of cellulose esters into elastomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Non-Reactive Compatibilizer in Cellulose Ester / Elastomer Compositions
[0097]Experiments were conducted to evaluate certain non-reactive compatibilizer in cellulose ester / elastomer compositions. In Table 1, the non-reactive compatibilizers evaluated are listed.
TABLE 1CompatibilizerCompoundMW1#CH22#EO3% EOMP4 ° C.Tergitol 15-S-957015963Tergitol 15-S-301400153085~50Polyethylene920321250~100block polyethyleneglycol5Polyethylene2250324080~85block polyethyleneglycolPolyethylene1400501650~100block polyethyleneglycol1Molecular Weight2Number of carbon atoms3Number of Ethylene Oxide groups4Melting Point5PE Block PEG
Tergitol 15-S-9 and Tergitol 15-S-30 are secondary alcohol ethoxylates obtained from Dow Chemical in Midland, Mich. The polyethylene block polyethylene glycol compatibilizers were obtained from Sigma-Aldrich. Although not wishing to be bound by theory, it is believed that the ethylene oxide units of the above compounds plasticizes the cellulose acetate butyrate and the hydrocarbon c...
example 2
Reactive Compatabilizers in Cellulose Ester / Elastomer Compositions
[0100]Reactive compatibilizers were evaluated to improve the mixing of CAB in styrene butadiene rubber (SBR). The reactive compatibilizers were selected such that they contained reactive groups that can react with the CAB and the rest of the molecule is compatible with the SBR. The molecular weight, and the type and concentration of the reactive moiety were varied.
TABLE 5BrandChemicalReactiveAcid number,NameCompositionManufacturerMoietyMwmg KOH / gmTm, ° C.CommentsSMAStyreneSartomer / CrayMaleic9500285180Styrene:MA =3000maleicValleyanhydride(Tm ~3:1anhydrideTg +copolymer55)EastmanMaleicEastmanMaleic4700015156G-3015anhydrideanhydridegraftedpolypropyleneEpoleneMaleicWestlakeMaleic1580045158E-43anhydrideChemicalsanhydridegraftedpolypropyleneLotaderRandomArkemaMaleic17100MaleicMAHterpolymer ofanhydrideanhydride8200Ethylene,~2.8 wt %Acrylic esterEsterand Maleic~6.5 wt %anhydrideLotaderRandomArkemaGlycidylNA65GlycidylGMA AXterp...
example 3
Use of Plasticizers
[0105]Masterbatches of cellulose esters with two different plasticizers at various loadings were prepared in an attempt to lower the Tg of the cellulose esters such that their flow temperature is lower than the typical rubber processing temperature of 150° C. Compounding in a Brabender mixer at 150° C. for 10 minutes at 100 rpm followed by cryogrinding yielded the masterbatches shown in Table 10.
TABLE 10Tg ofTg ofMasterCE,QuantityType ofQuantity ofmasterBatchCE1° C.of CE, gPlasticizerplasticizer, gBatch, ° C.MB 1CAB 551-0.2101100Eastman 16821084MB 2CAB 553-0.4136100Eastman 1682585MB 3CAB 381-0.1123100Eastman 1682087MB 4CAB 381-2133100Eastman 1682595MB 5CAB 553-0.4136100Poly (ethylene2565glycol)3MB 6CAB 381-2133100Poly (ethylene2570glycol)MB 7CAP 504-0.2159100Poly (ethylene3093glycol)MB 8CAP 482-0.5142100Poly (ethylene2590glycol)MB 9CA 398-3180100Poly (ethylene40109glycol)1CE—Cellulose Ester2bis(2-ethylhexyl)-1,4-benzene dicarboxylate3polyethylene glycol - molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Specific volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com