Porous implant structures
a technology of porous implants and implants, applied in the field of porous implants, can solve the problems of insufficient strength to serve as weight-bearing structures in many medical implants, formation of undesirable metal compounds in metal foam, and the conventional metal foam fabrication process consumes substantial amounts of energy, so as to improve strength and porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
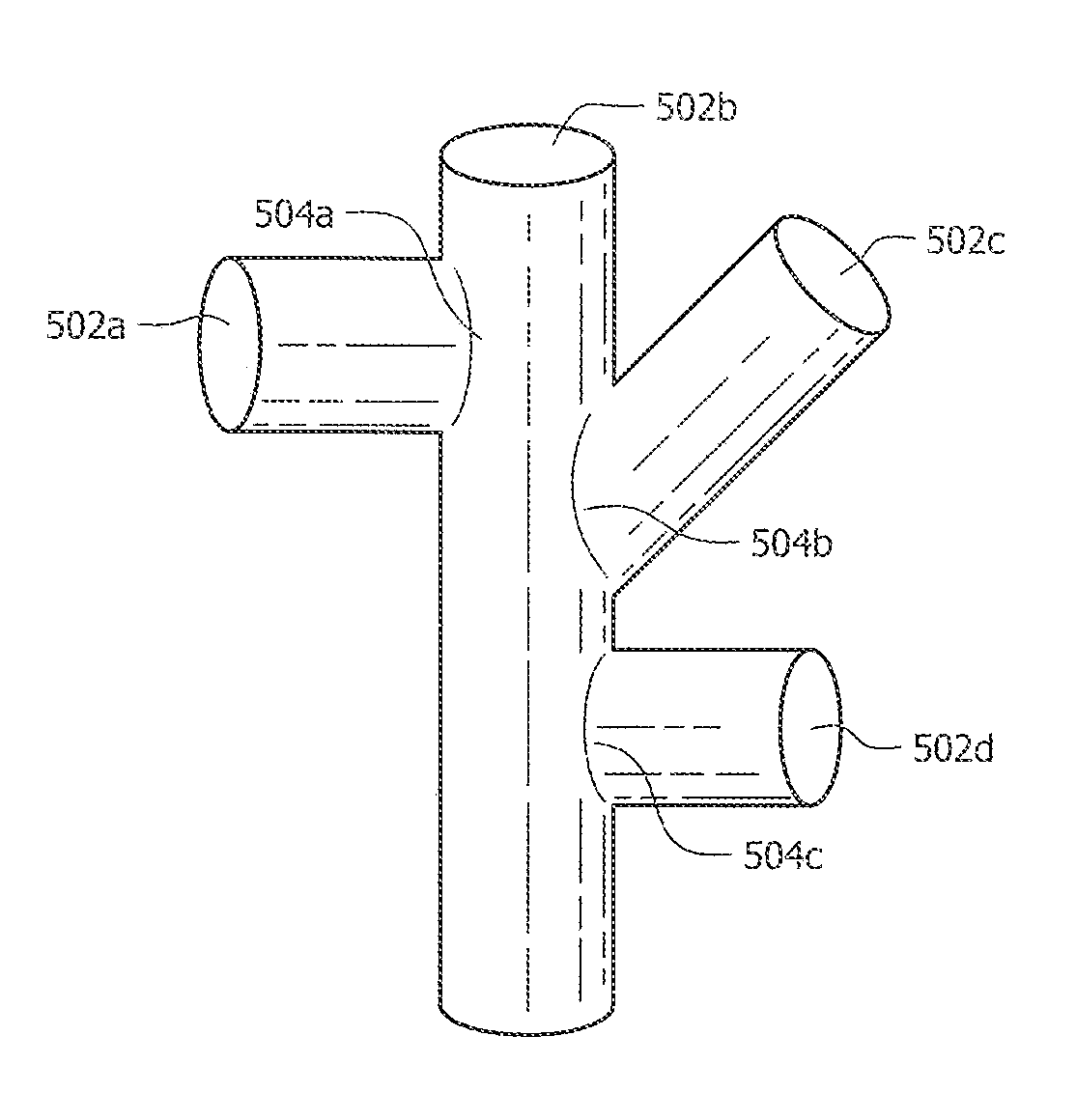
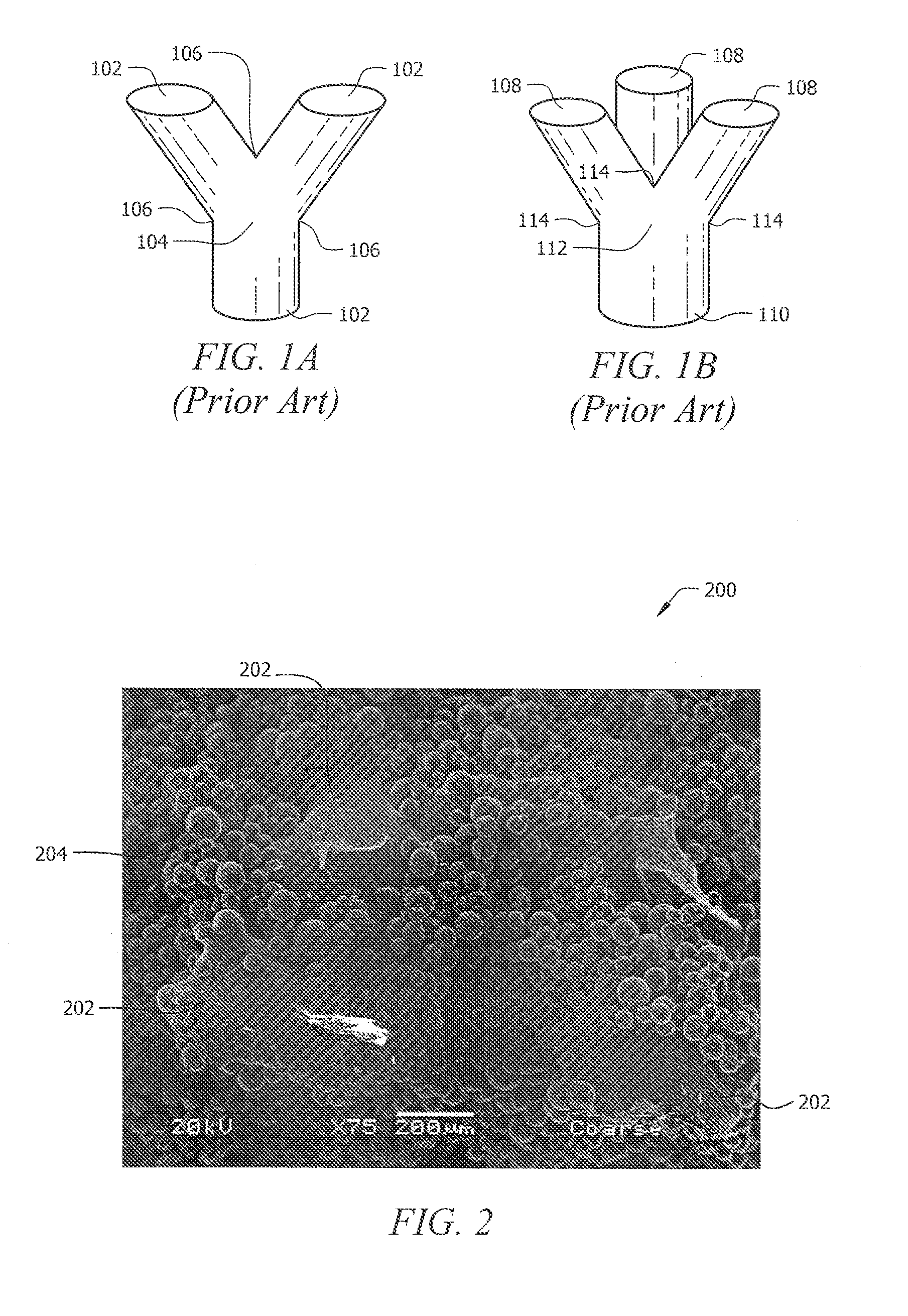
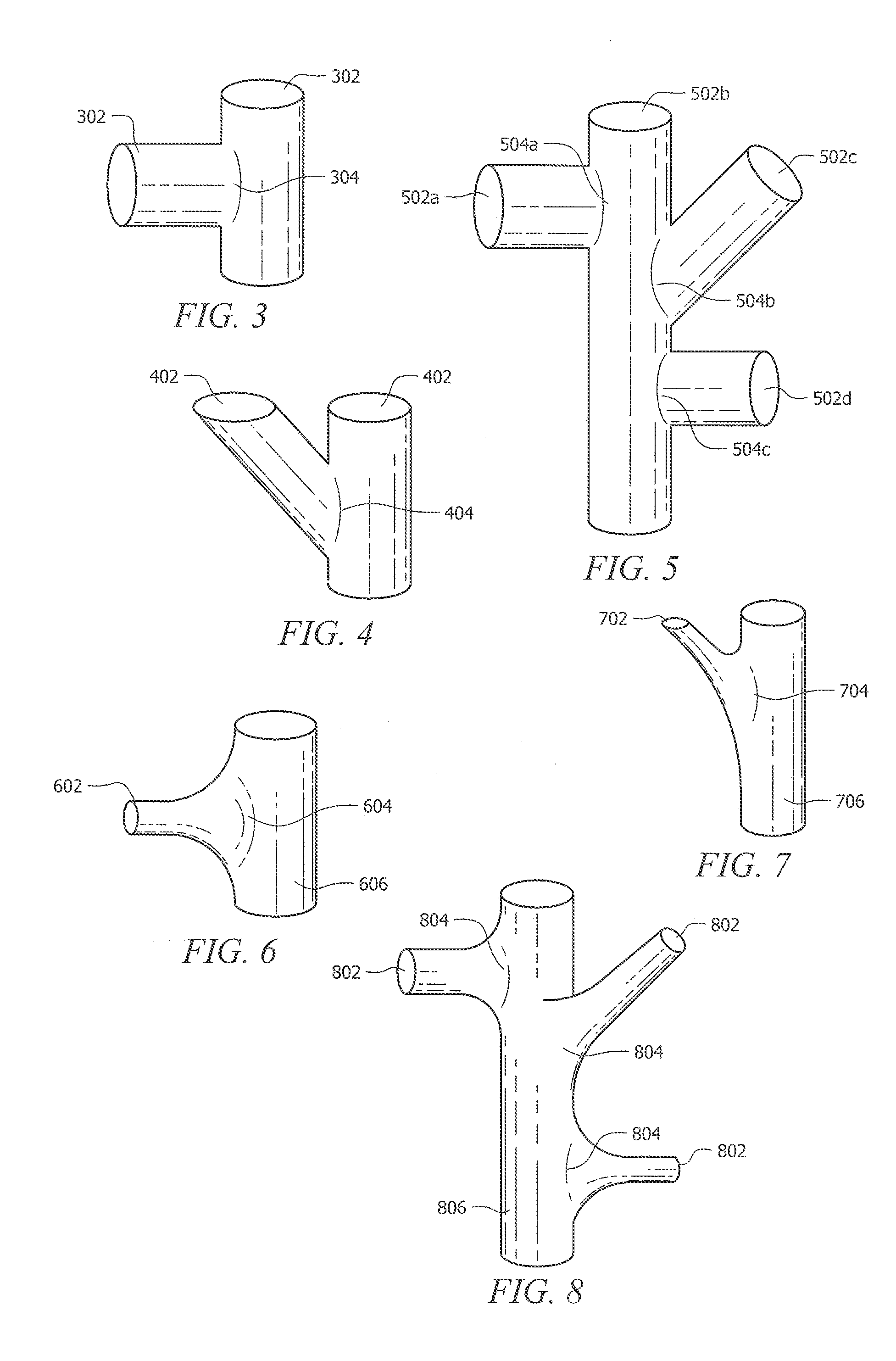
[0086]As discussed above, Rapid Manufacturing Techniques (RMT) such as Direct Metal Fabrication (DMF) can be used to produce porous structures for medical implants. However, using DMF or other RMT to fabricate porous structures can create weak areas between fenestrations of the three-dimensional porous structure. This is mostly due to the shapes and configurations of the cells that have been used in the prior art to form these porous structures. In particular, fractures typically occur at areas where struts are connected together at a node. The fractures occur in porous structures of the prior art because the cross-sectional area of a strut where it connects to the node is typically less than the cross-sectional area of the resulting node. The areas where the struts connect to their node, typically referred to as stress risers, are common points of structural failure. The pattern of failure at the stress risers can also occur when the molten phase of particles does not completely me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosities | aaaaa | aaaaa |
| porosities | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com