Selective fracture face dissolution
a fracture face and selective technology, applied in the direction of fluid removal, borehole/well accessories, chemistry apparatus and processes, etc., can solve the problems of acid corrosion creating iron compounds such as iron chlorides, strong acids corroding such materials, and affecting the stability or effectiveness of other components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
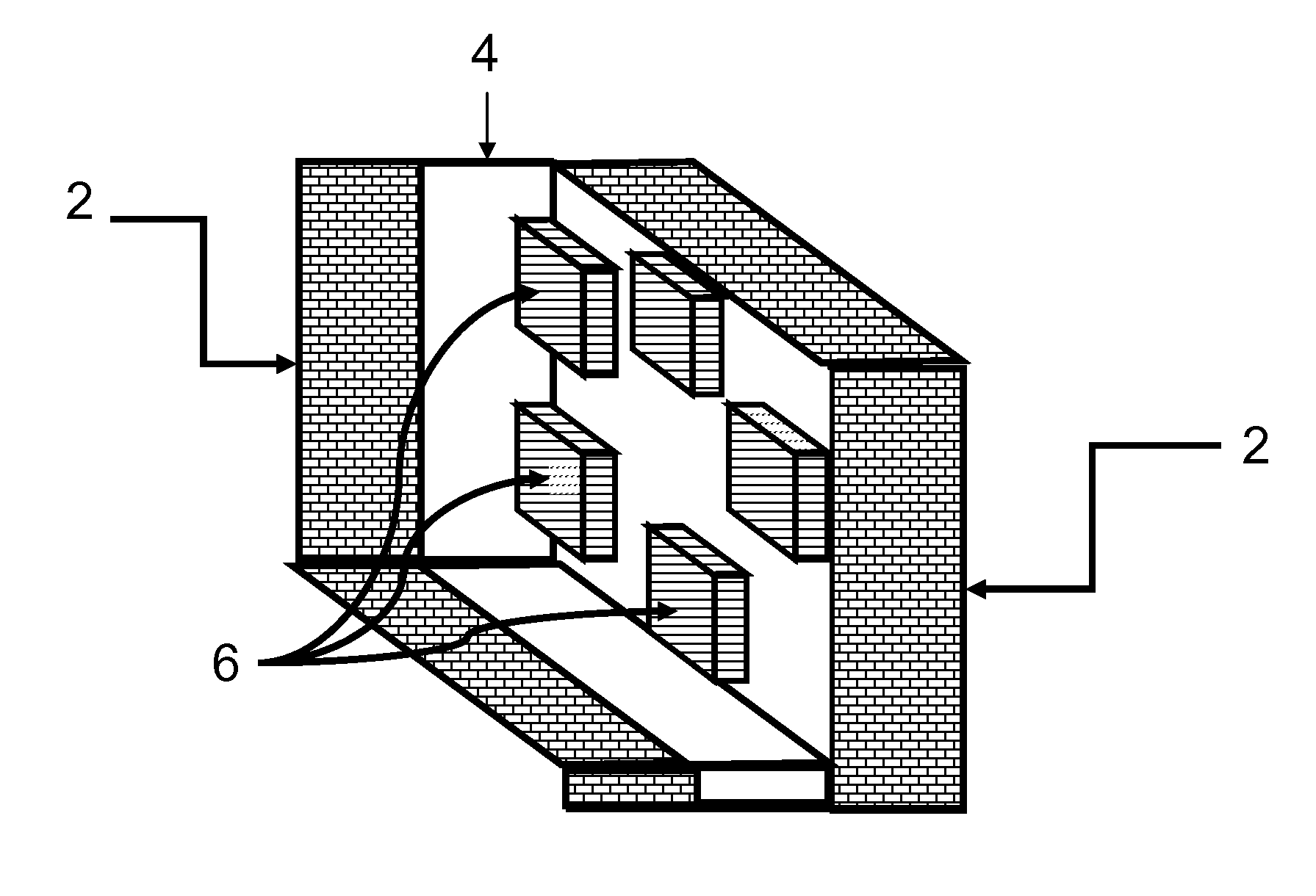
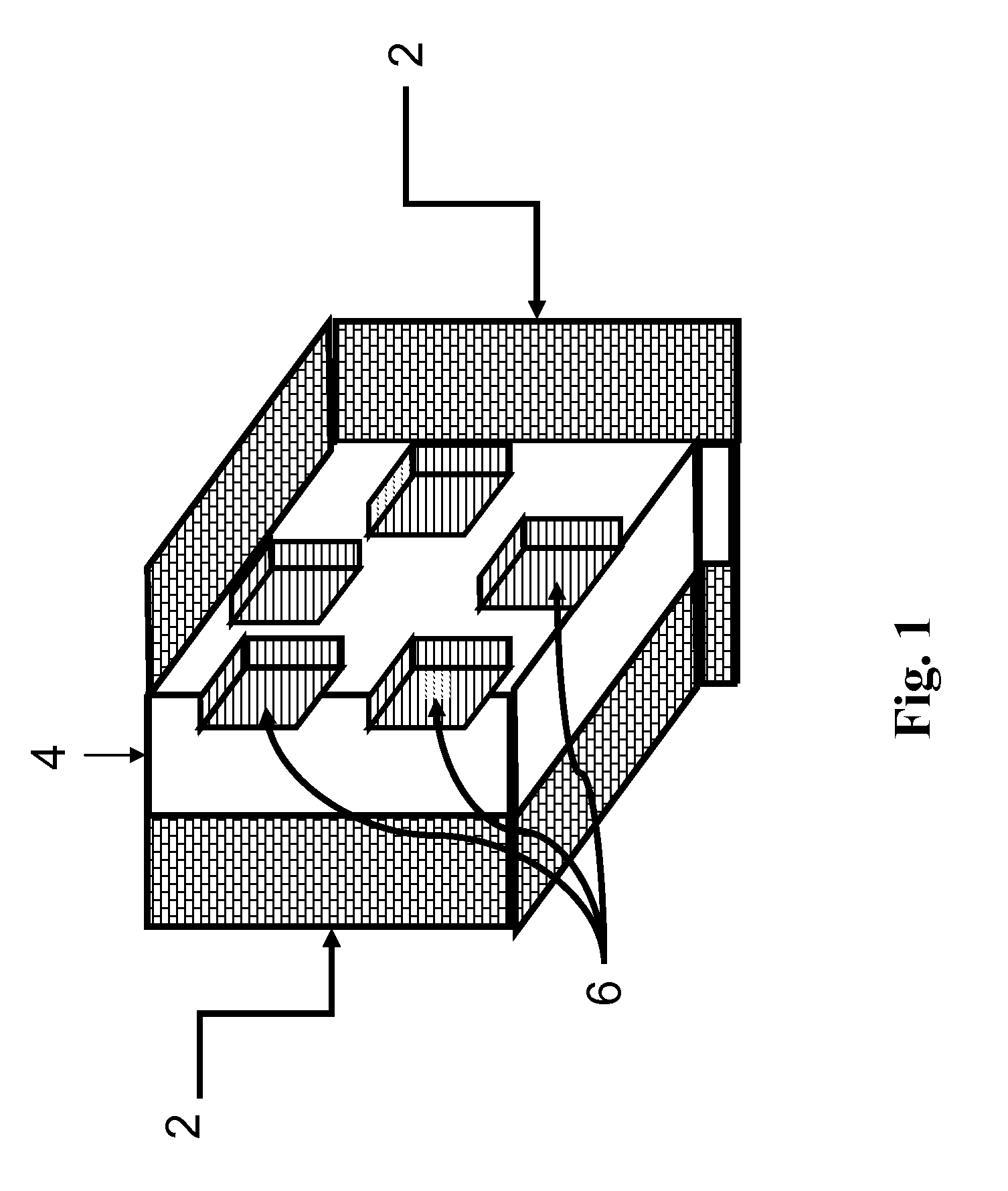
[0042]FIG. 1 shows a schematic of how a fracture would appear if created by the method of the invention. The fracture [4] in the formation [2] contains regions [6] that are not open to fluid flow. These regions are where the inert or reactive masking material is trapped when the fracture closes. The fracture face is protected from the formation dissolving agent at those locations.
example 2
[0043]A) The following is a representative job design that would be used for a typical treatment with a mixture of solid acid precursor fibers and solid acid precursor beads. The base fluid is 2.4 g / L of zirconium crosslinked CMHPG.
Solid AcidSolid AcidSolid AcidSolid AcidStagePrecursorPrecursorPrecursorPrecursorRateVolumeBeadsBeadsFiberFiberStagekL / minBase FluidkLkg / Lkgg / LkgPAD3.98Z-CMHPG159000013.98Z-CMHPG11.40.066802.42723.98Z-CMHPG11.40.1213612.42733.98Z-CMHPG11.40.1820414.85443.98Z-CMHPG11.40.2427224.85453.98Z-CMHPG11.40.3034027.28263.98Z-CMHPG11.40.3640827.28273.98Z-CMHPG11.40.4247639.610983.98Z-CMHPG11.40.4854439.6109FLUSH3.98Water46.50000[0044]B) The following is a representative job design that would be used for a typical treatment with solid acid precursor beads and an inert masking material. The base fluid is 4.2 g / L of guar crosslinked with boric acid.
Solid AcidSolid AcidInertInertStagePrecursorPrecursorMaskingMaskingRateVolumeBeadsBeadsMaterialMaterialStagekL / minBase Flu...
example 3
[0046]A pack that was a mixture of polylactic acid beads and rubber pellets was sandwiched between the ends of two carbonate cores. Both cores were saturated with 2% KCl brine and a small amount of water was added to the pack to reduce the amount of air trapped in the pack. The complete combination of pack and cores was then heated to 135° C. for 4 hours. Upon disassembly, and inspection of the surface of the cores, it was seen that there were areas where the rubber pellets had agglomerated; these areas were not etched but the remainder of the surface was etched.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com