Compositions and methods for treatment of peripheral vascular disease
a technology for vascular disease and compositions, applied in the field of compositions and methods for treating peripheral vascular disease, can solve the problems of disappointing clinical trials to alleviate ischemia, no one can reverse the disease process and directly repair lasting damage, etc., to achieve improved limb function, improve treatment effect, and improve treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Angiotensin (1-7) Treatment in an Animal Model of Chronic Hind Limb Ischemia Improved Blood Flow and Limb Function
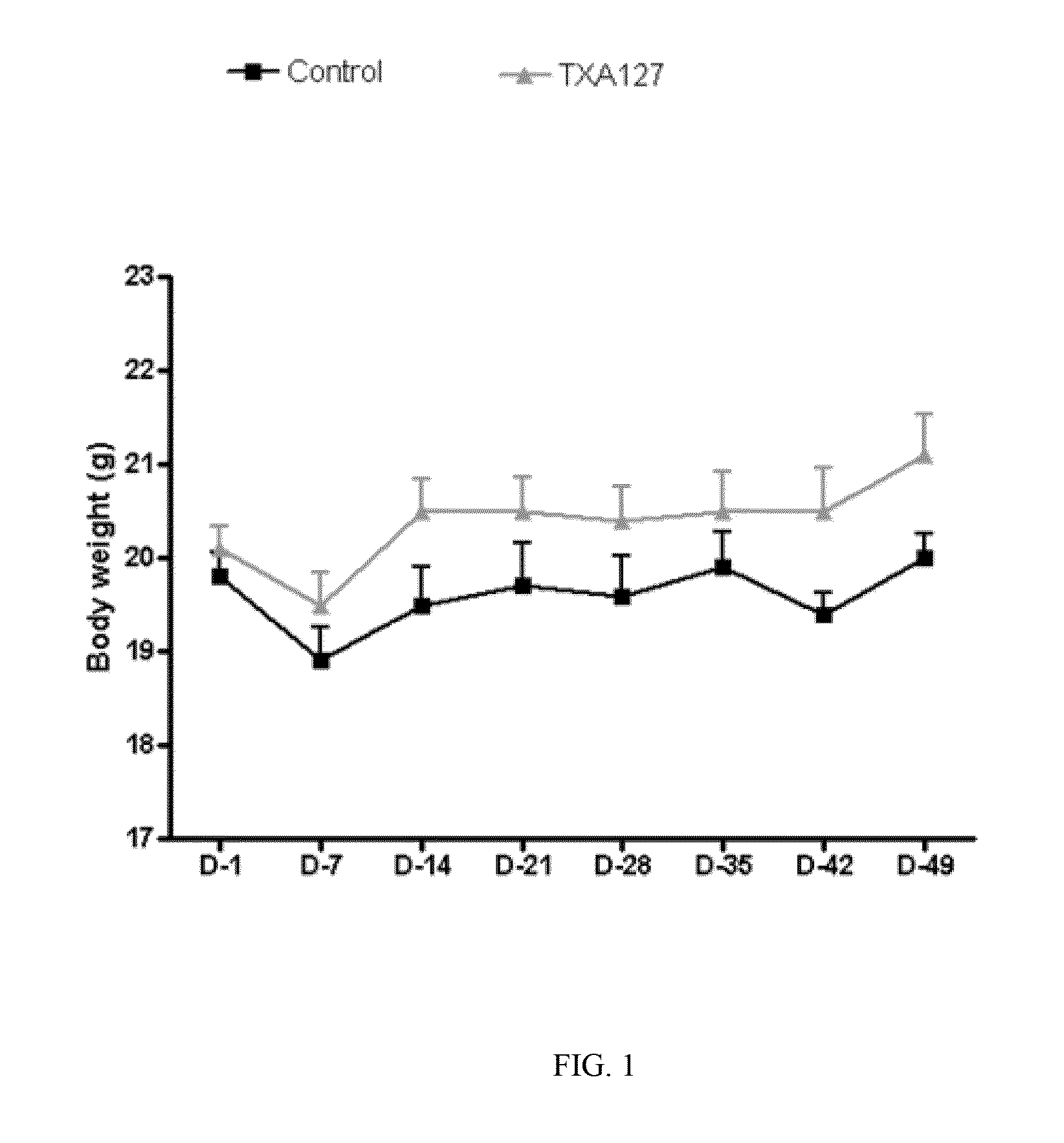
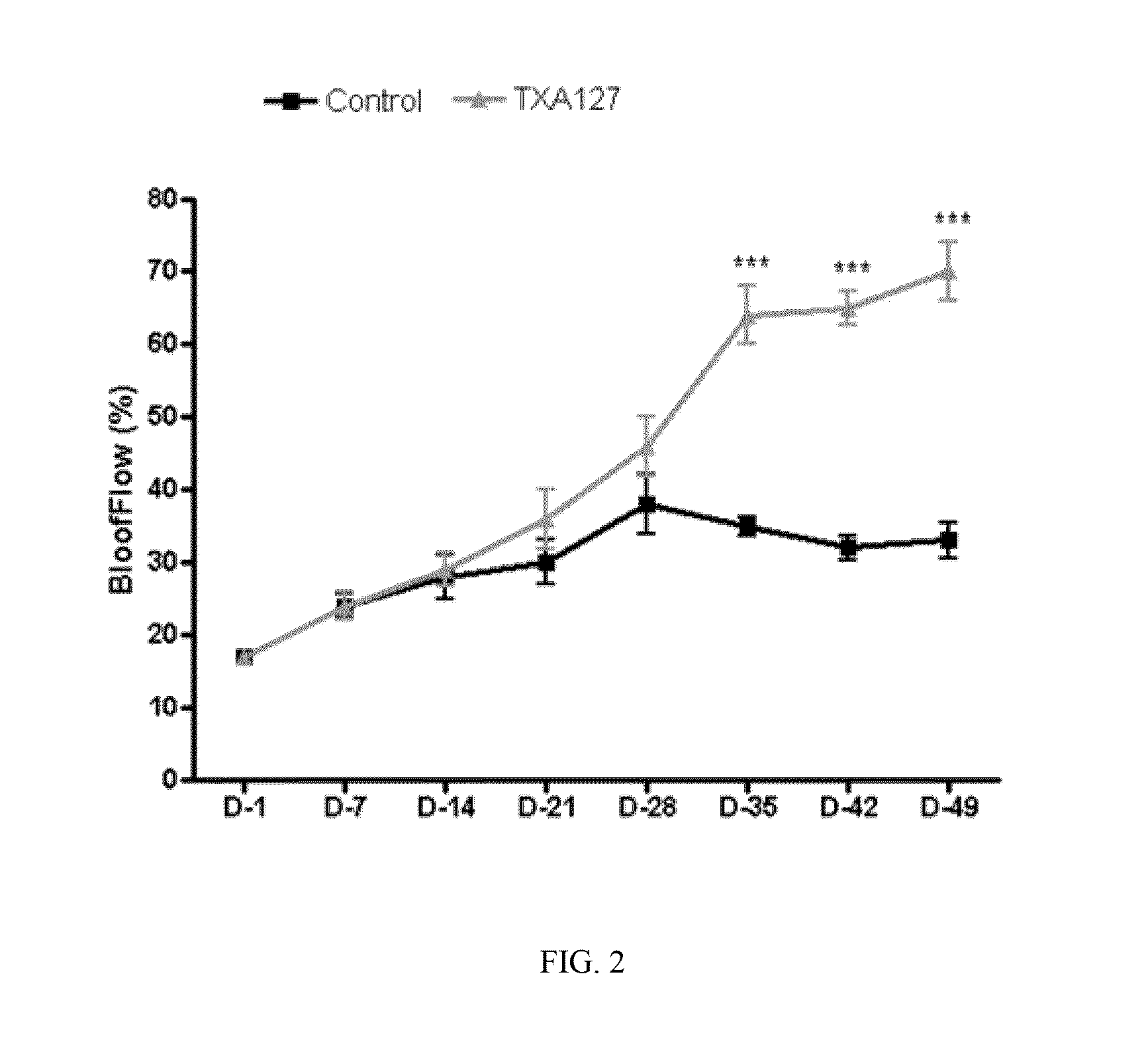
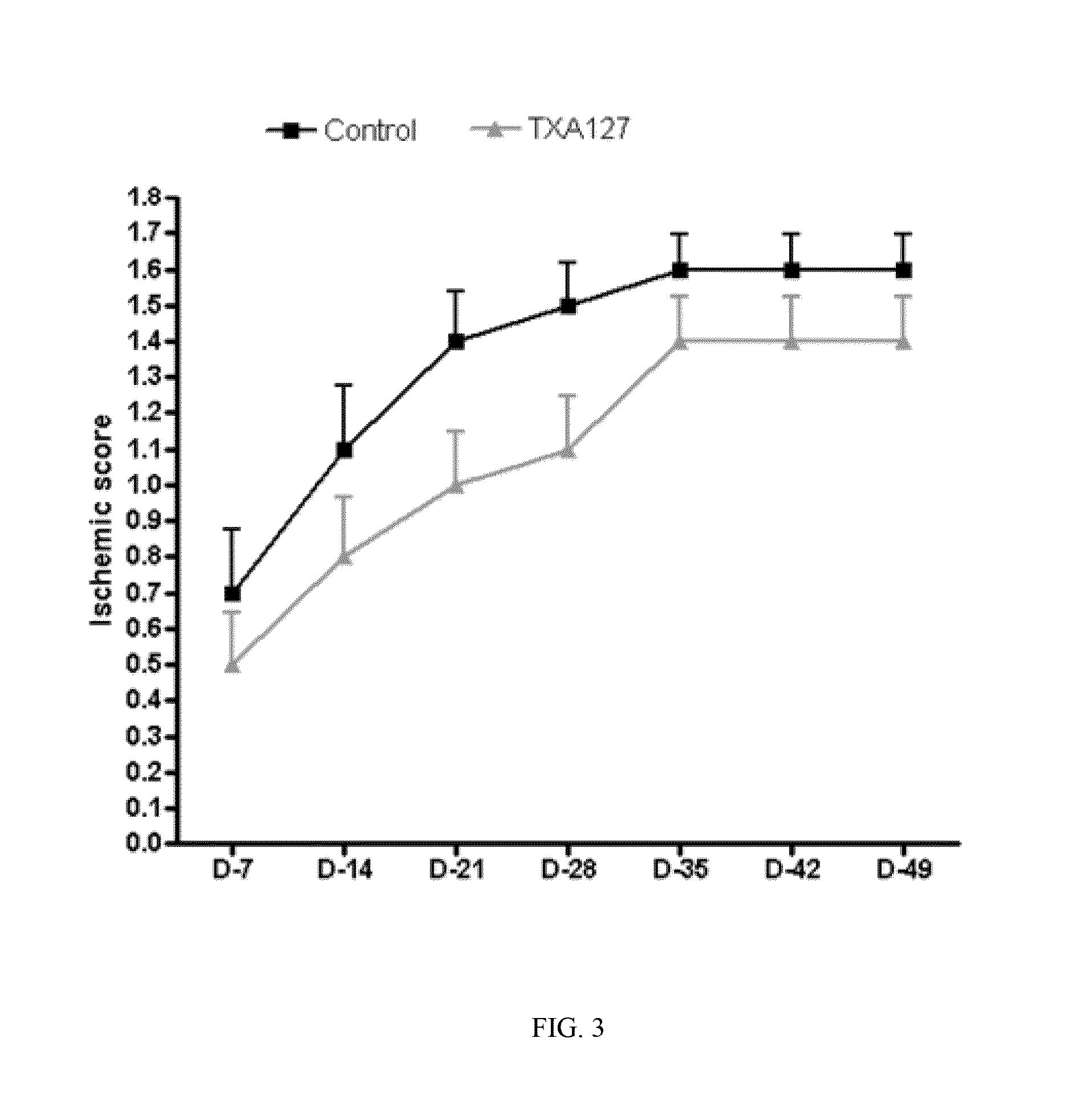
[0231]The present Example demonstrates that angiotensin (1-7) can be used to effectively treat ischemic diseases. In this example, a linear angiotensin peptide TXA127 having an amino acid sequence of Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7 (SEQ ID NO: 1) was used as an example to assess the therapeutic effect of angiotensin (1-7) in a mouse hind limb ischemia model.
[0232]Hind Limb Ischemia Model
[0233]A stable hind limb ischemia model has been described previously and is generally characterized by uniform ischemic damage useful for examining the effect of various therapies (Goto, et al. Tokai J Exp Clin Med, 31(3):128 2006; Kang Y, et al. PLoS One. 2009; 4(1):e4275)). The hind limb ischemia model in mice used in this example involves two ligations of the proximal end of the femoral artery and its dissection between the two ligatures. The surgery causes obstruction of the blood...
example 2
PanCyte Treatment in an Animal Model of Chronic Hind Limb Ischemia Improved Blood Flow and Limb Function
[0261]The present Example demonstrates that PanCyte can be used to effectively treat ischemic diseases. In this example, a cyclic angiotensin peptide having an amino acid sequence of Asp1-Arg2-Val3-Ser4-Iles-His6-Cys7 (SEQ ID NO:22) was used as an example to assess the therapeutic effect of PanCyte in a mouse hind limb ischemia model.
[0262]A total of 49 female mice were utilized, divided into three groups: 16 in group 1F, 17 in group 2F and 16 in group 3F. The number of the groups and the total number of animals was based on previous studies demonstrating that this was the minimum number of animals per group sufficient to obtain indicative / significant information. Table 13 shows the design of each group.
TABLE 13Group DesignSurgicalTreatmentDoseRoute ofGroupProcedure(Lot)mg / kgVolumeAdministration1F✓NegativeNA5 ml / kgSC(N = 16)control(vehicle)2F✓PanCyte500 μg / kg5 ml / kgSC(N = 17)3F✓Pa...
example 3
Lower Dose PanCyte and Continuous Infusion Treatments in an Animal Model of Chronic Hind Limb Ischemia Improved Blood Flow and Limb Function
[0291]The present Example demonstrates that doses of PanCyte between 1 μg / kg and 50 μg / kg can be used to effectively treat ischemic diseases. In this example, a cyclic angiotensin peptide having an amino acid sequence of Asp1-Arg2-Val3-Ser4-Ile5-His6-Cys (SEQ ID NO:22) was used to assess the therapeutic effect of PanCyte in a mouse hind limb ischemia model.
[0292]A total of 98 female mice were utilized, divided into three groups: 15 in group 1F, 17 in group 2F, 17 in group 3F, 16 in group 4F, 17 in group 5F, and 16 in group 6F. The number of the groups and the total number of animals was based on previous studies demonstrating that this was the minimum number of animals per group sufficient to obtain indicative / significant information. Table 18 shows the design of each group.
TABLE 18Group DesignRoute ofSurgicalDoseAdminis-GroupProcedureTreatmentm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com