6-AMIDO DERIVATIVES OF 4, 5-a EPOXYMORPHINANS FOR THE TREATMENT OF PAIN
a technology of epoxymorphinans and derivatives, applied in the field of 6amido derivatives of 4, 5aepoxymorphinans, can solve the problems of life-threatening respiratory depression in patients and unwanted peripheral side effects, and achieve the effect of lessening the liability of constipation and respiratory depression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
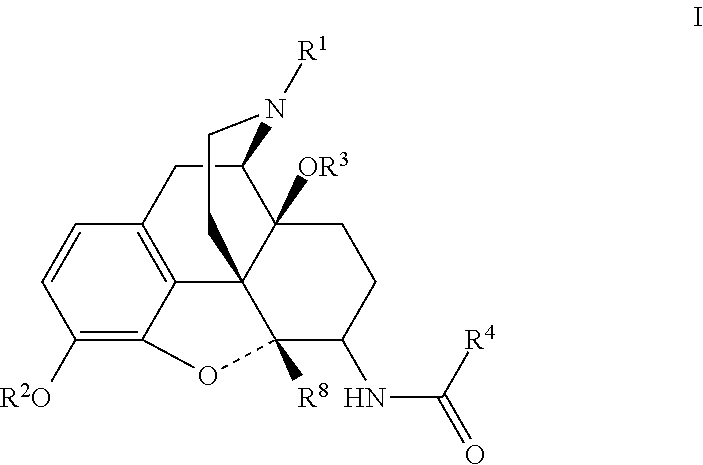
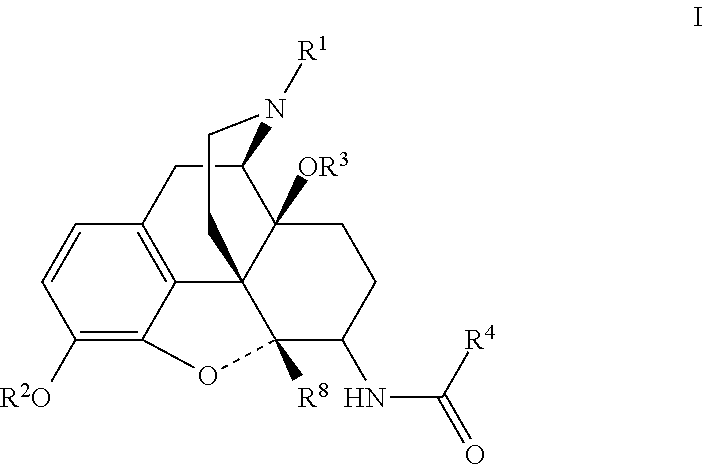
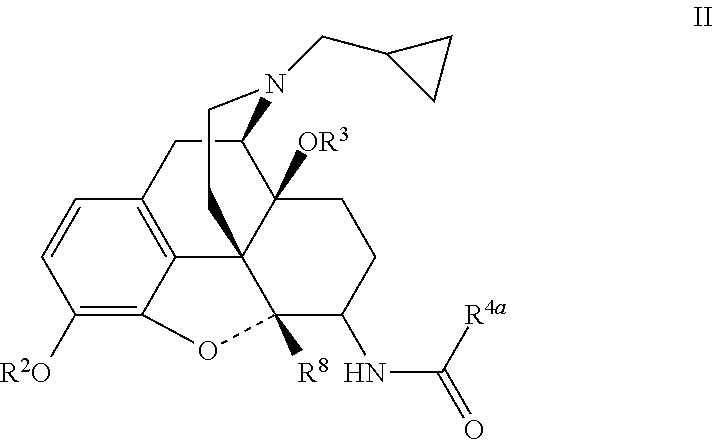
[0023]Analgesic compounds of the invention fall into two primary classes: compounds of general formula II, in which R1 is cyclopropylmethyl, and compounds of general formula I, in which R1 is not cyclopropylmethyl. The compounds of general formula I include a series in which R1 is allyl and one in which R1 is cyclobutylmethyl. When R1 is —CH2-Het, Het may be tetrahydrofuranyl.
[0024]In one aspect, the invention relates to compounds of formula I:
[0025]Some embodiments of the invention can be represented by the formula:
which is a subset of formula I. In these compounds, R1 is cyclobutylmethyl or allyl;
R2 is chosen from hydrogen, (C1-C6)acyl, (C1-C6)oxaalkyl, and (C1-C6)acyloxaalky;
R3 is hydrogen or methyl;
R4 is chosen from
(a) phenyl substituted at other than 2 or 6 with from one to three substituents chosen from bromo, chloro, iodo, hydroxy, nitro, cyano, (C1-C3)alkyl, (C1-C3)haloalkyl, (C1-C3)haloalkoxy, (C1-C3)alkoxy and R10;
(b) optionally substituted naphthylene;
(c) optionally subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| physical dependence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com