Compositions and methods of using (r)-pramipexole
a technology of r-pramipexole and compositions, applied in the field of compositions and methods of using r-pramipexole, can solve the problems of limiting the usefulness of the neuroprotective potential of r-pramipexole, requiring dose titration and dose limitation, and limited doses both temporally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Measurement of the Dopamine Receptor Affinities for the R(+) and S(−) Enantiomers of Pramipexole
[0275]The S(−) enantiomer of pramipexole has historically been characterized as a high affinity dopamine receptor ligand at the D2 (both the S and L isoforms), D3 and D4 receptors, although the highest affinity is seen for the D3 receptor subtype. The dopamine receptor ligand affinity of (S)-pramipexole and of (R)-pramipexole from journal publications has been tabulated (data are reproduced in Table 10). Although the conditions under which each study or experiment was carried out are slightly different, and different radio-ligands were used, the data show comparable affinities for the various dopamine receptors. Studies we conducted on the dopamine receptor affinities of the S(−) and the R(+) enantiomers of pramipexole are also shown in Table 10. These data demonstrate an unexpectedly large difference in the affinities of the two enantiomers of pramipexole for all dopamine receptors. Tabl...
example 2
[0278]In vivo studies to determine the MTD and NOAEL in dogs for 100% pure preparations of the R(+) and S(−) enantiomers of pramipexole, and a mixture (R 99.5% / S 0.5%). The form of (R)-pramipexole was (R)-pramipexole dihydrochloride monohydrate.
[0279]The following in vivo study in beagle dogs was undertaken to test the hypothesis that the large observed difference in receptor binding affinities for the R(+) and S(−) enantiomers of pramipexole will translate to a large observed difference in the observed maximum tolerated dose (MTD) and / or no observable adverse effect level (NOAEL) of the two enantiomers. Dogs were administered preparations of each enantiomer prepared as a highly purified compound (100% pure preparations (within the limits of analytical detectability)), or a preparation of the R(+) enantiomer contaminated by 0.5% of the S(−) enantiomer of pramipexole.
[0280]Three groups of four non-naïve male beagle dogs were used in the study. Each group was administered various dose...
example 3.1
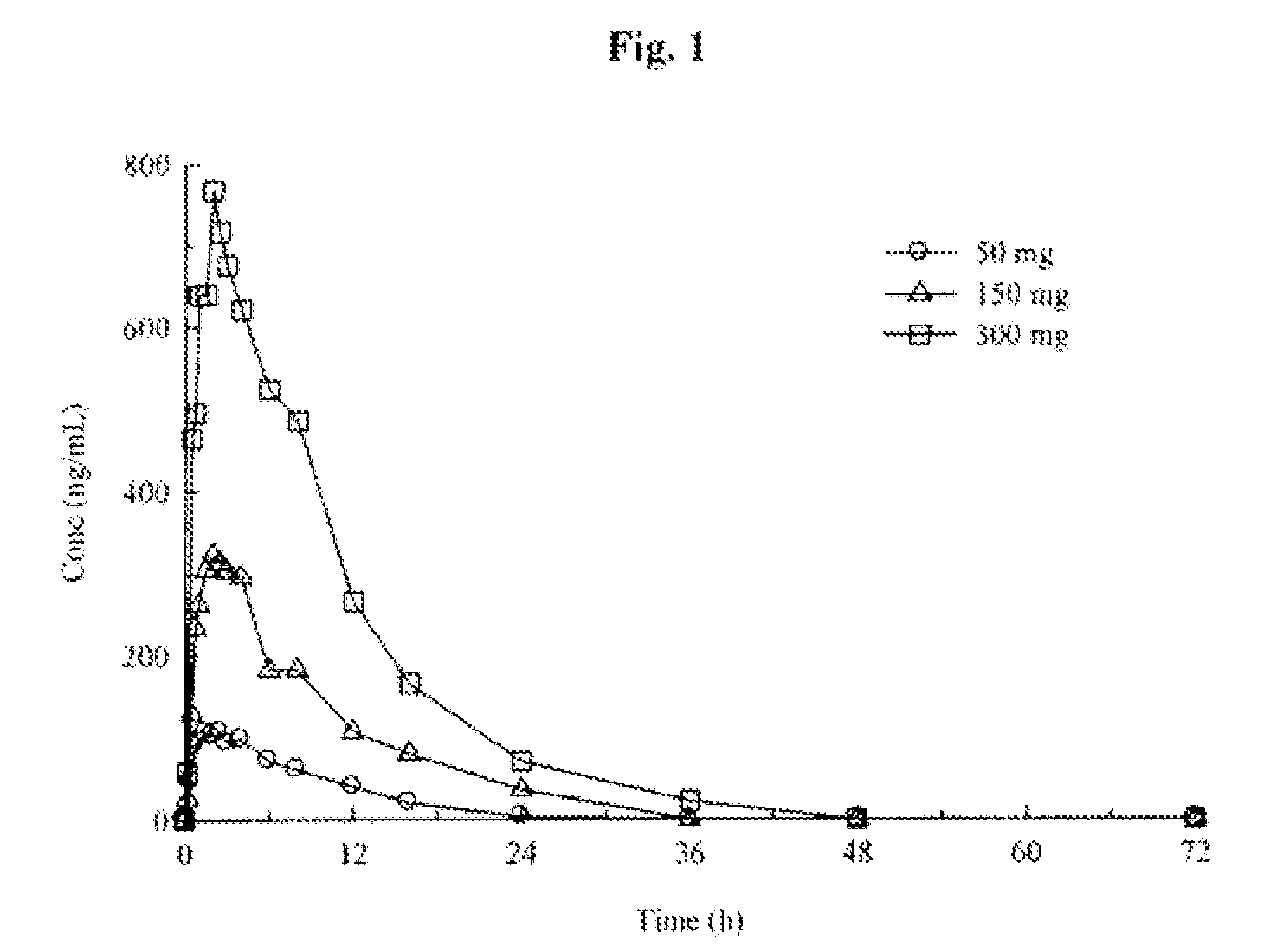
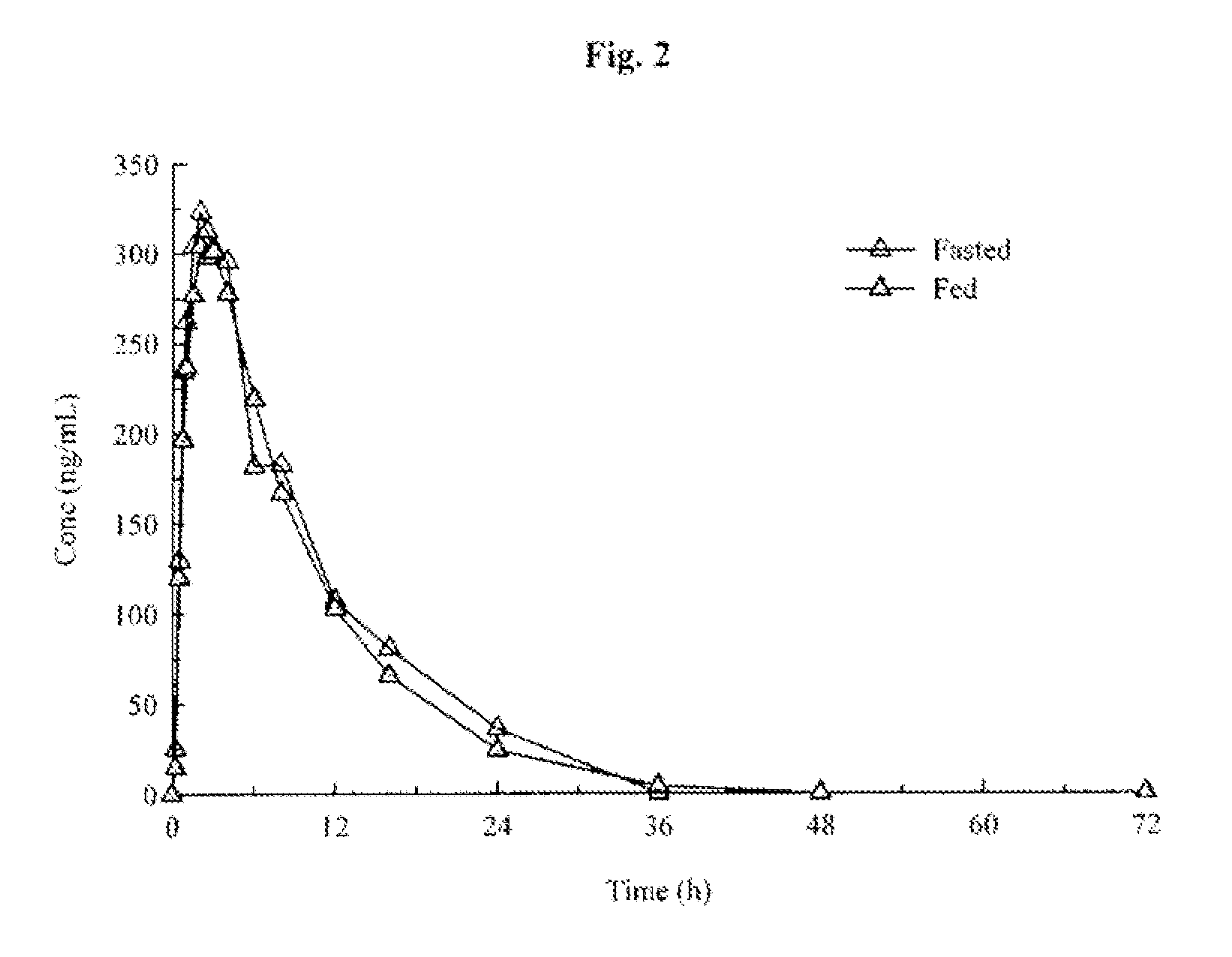
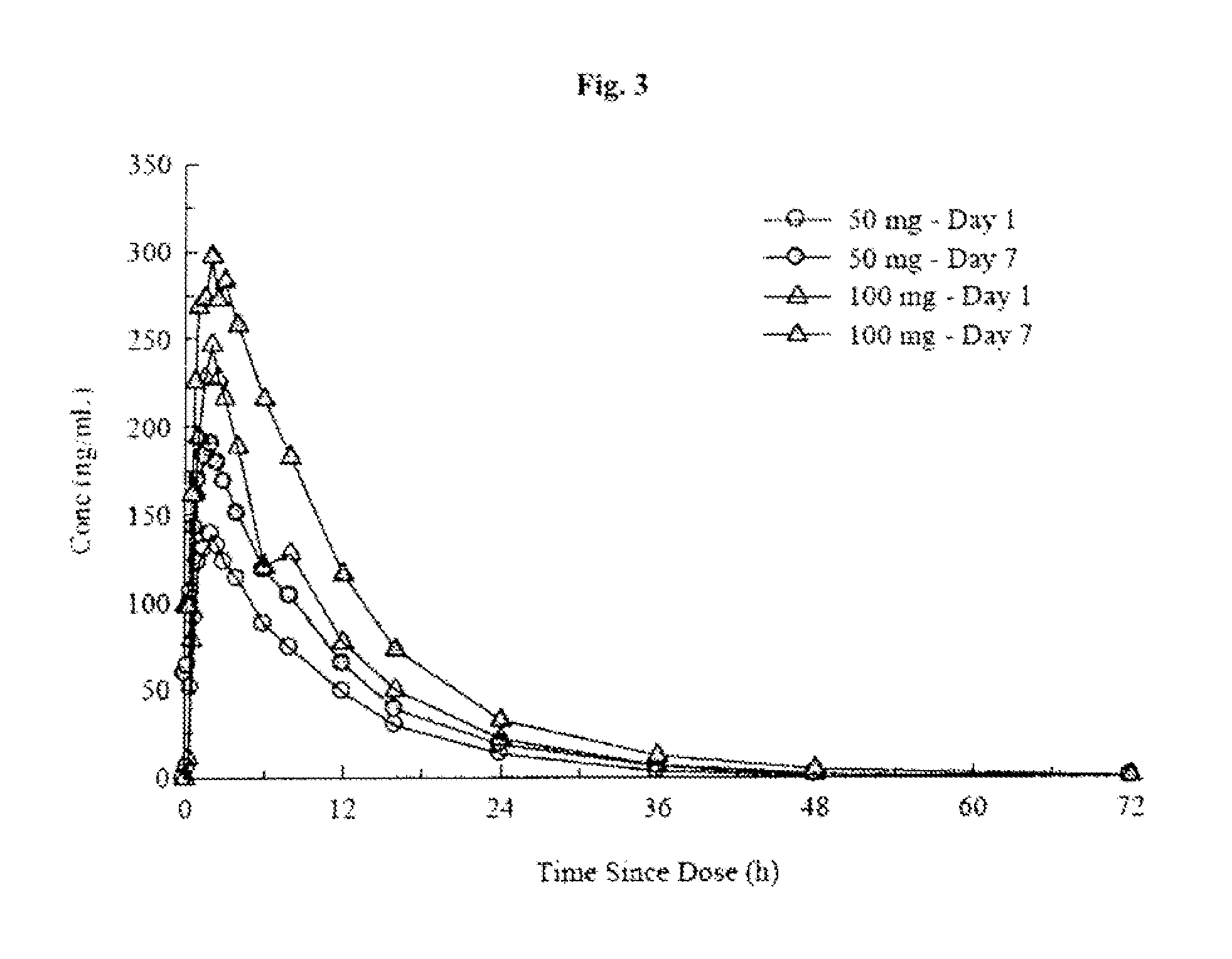
[0284]Toxicology studies in rats and minipigs and Phase I studies in healthy adult volunteers. Two-week and three-month toxicology studies of (R)-pramipexole in rats and minipigs were completed. NOAEL dose levels of 150 mg / kg at two-weeks and 100 mg / kg at three-months for rats and 75 mg / kg at two-weeks and 50 mg / kg at three-months for minipigs were established. Phase I studies of healthy adult volunteers have demonstrated that (R)-pramipexole in ascending single doses up to 300 mg and multiple doses up to 200 mg per day for 4½ days is safe and well-tolerated. The Mirapex® label specifies a starting dose of 0.125 mg and a maximum total daily dose of 4.5 mg. The Phase I data demonstrate, therefore, that (R)-pramipexole may be safely administered (1) at starting doses that are at least 2400-fold higher than the Mirapex® starting dose and (2) at steady state doses that are at least 44-fold higher than the highest recommended dose of Mirapex®. The form of (R)-pramipexole was (R)-pramipex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com