Methods and compositions for treating schizophrenia
a composition and schizophrenia technology, applied in the field of compositions for treating schizophrenia or bipolar disorder, can solve the problems of ineffective treatment of schizophrenia patients with current antipsychotics, etc., and achieve the effect of preventing or slowing the progression of cognitive impairment or bipolar disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
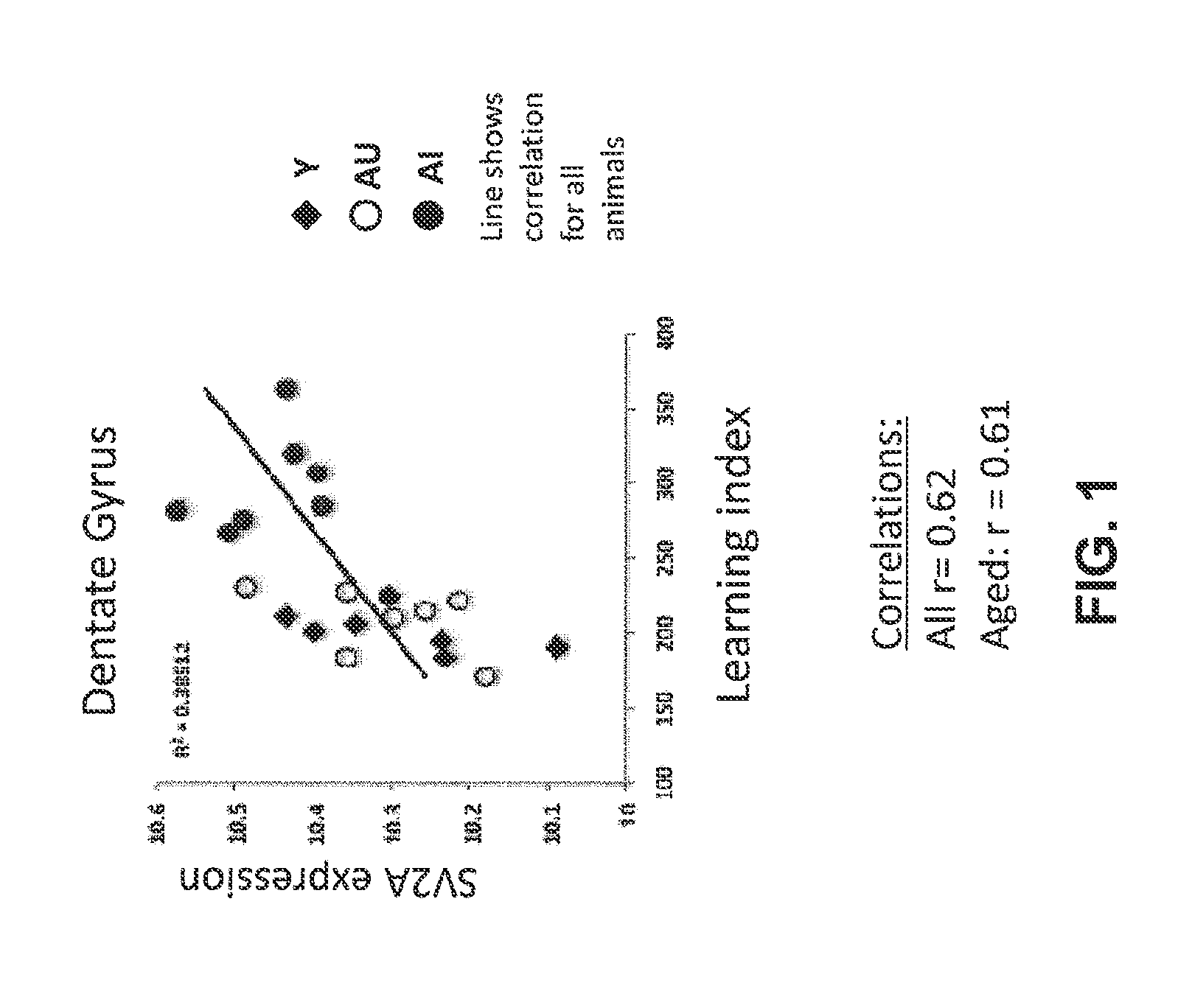
Increased Gene Expression of SV2A in Aged-Impaired Rats
Behavioral Characterization of Young, Aged-Impaired and Aged-Unimpaired Rats in Morris Water Maze (MWM)
[1870]Behavioral tests were performed on young (4 months old) and aged (24 months old) pathogen-free male Long-Evans rats.
[1871]The MWM apparatus consists of a large, circular pool (diameter 1.83 m; height, 0.58 m) filled with water (27° C.) that is made opaque through the addition of non-toxic pigment or some other substance. In the typical “hidden platform” version of the test, rats are trained to find a camouflaged white escape platform (height, 34.5 cm) that is positioned in the center of one quadrant of the maze about 1.0 cm below the water surface. This platform can be retracted to the bottom of the tank or raised to its normal position from outside the maze during behavioral testing. The location of the platform remains constant from trial to trial. Because there are no local cues that mark the position of the platform, ...
example 2
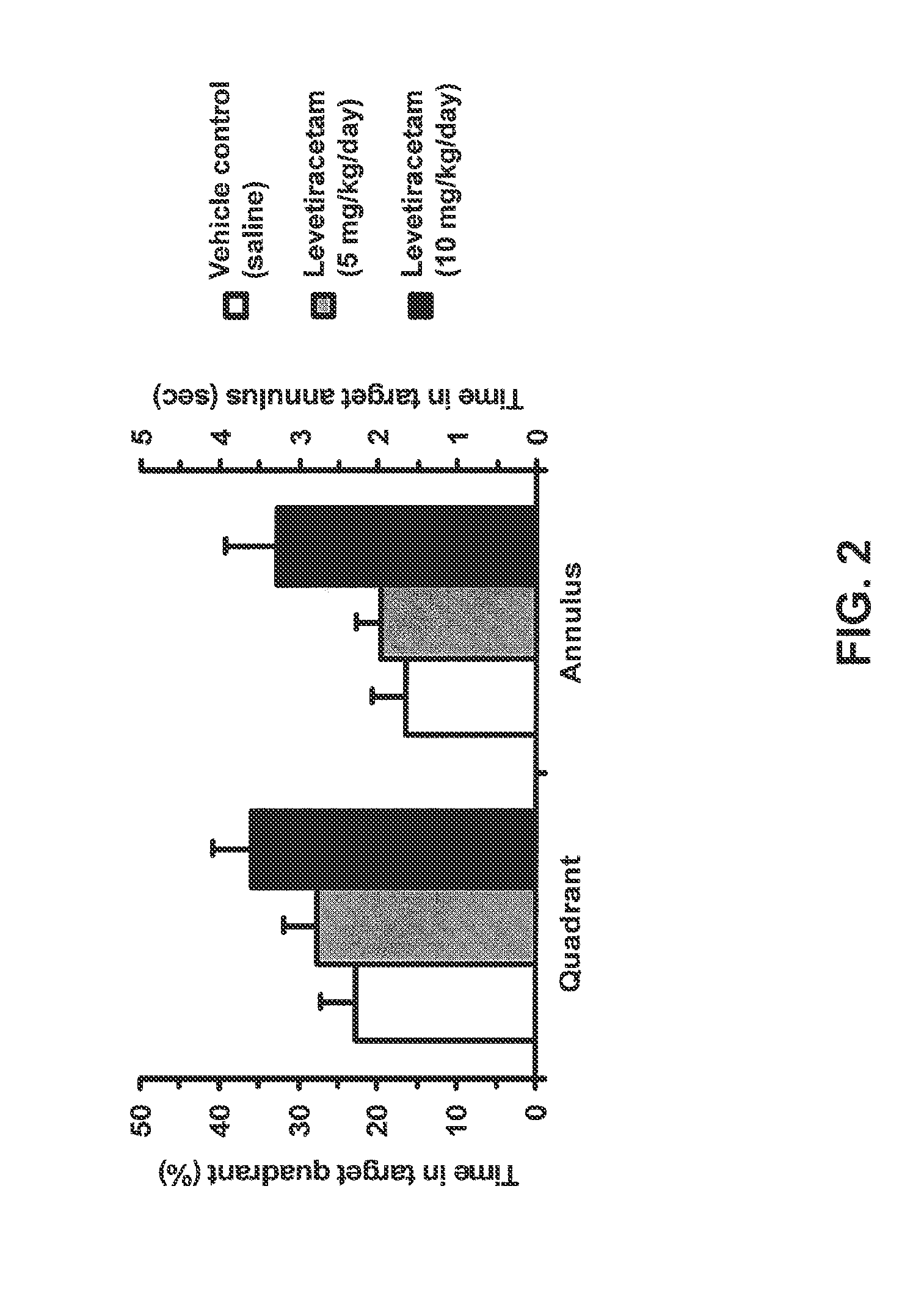
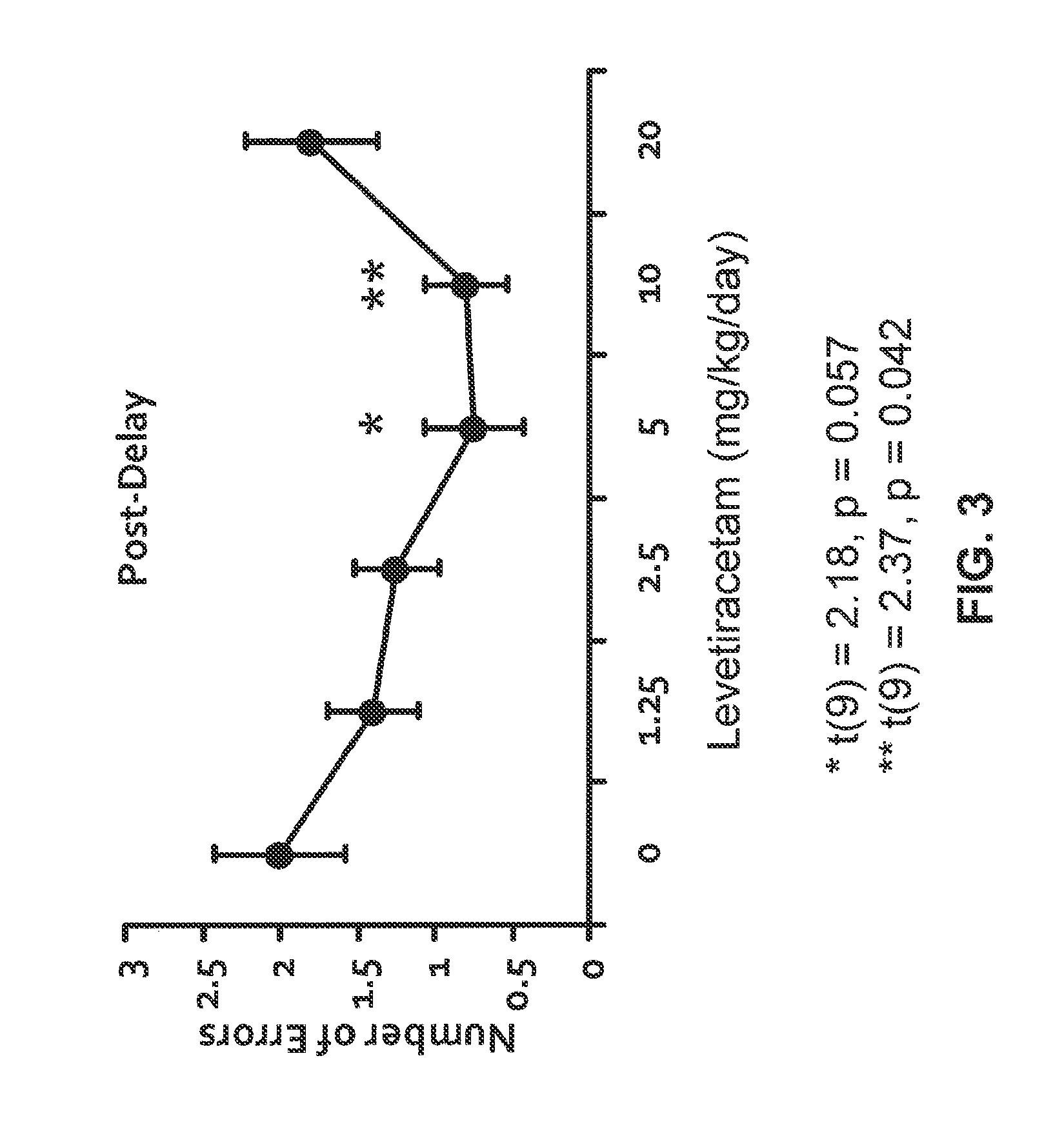
Effect of Levetiracetam in Aged-Impaired Rats
[1888]Morris Water Maze Results
[1889]Six Age-Impaired (AI) Long-Evans rats (as characterized above) were tested for their memory of new spatial information in the MWM, under different drug / control treatment conditions (vehicle control and two different dosage levels of levetiracetam). The MWM protocol was substantially the same as the one described in Example 1. Specifically for this study, a retention trial was performed after the training trials, as described below.
[1890]AI rats were given six training trials per training day with a 60-sec inter-trial interval between each training trial for two consecutive days. On each training trial, the rat was released in the maze from one of four equally spaced starting positions around the perimeter of the pool. If the rat did not locate the escape platform within 90 sec on any trial, the experimenter guided the rat to the platform, where it remained for 30 sec. 30 minutes to 1 hour prior to all ...
example 3
Effect of Levetiracetam in Human Subjects with aMCI
[1903]A within-subjects trial of 8 weeks duration, involving 17 amnestic MCI (aMCI) subjects and 17 age-matched controls with a low dose treatment of levetiracetam is conducted. During the course of the study, each aMCI subject receives both drug and placebo treatments separately in two periods of two weeks each, with the order of treatments among different aMCI subjects counterbalanced (see FIG. 7). Age-matched control subjects treated with placebo serve as a further control. Cognitive testing and fMRI imaging data are obtained from the subjects after each two week period of drug / placebo treatment.
Participants and Clinical Characterization
[1904]17 right-handed aMCI patients are recruited from the Alzheimer's Disease Research Center (ADRC) at the Johns Hopkins Hospital and other referrals. An additional 17 right-handed healthy volunteers are recruited from the pool of control participants in the ADRC and other referrals. All partici...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com