Method of determining clonotypes and clonotype profiles
a clonotype and clonotype technology, applied in the field of determining can solve the problems of limited natural variability predictability and challenges in the efficient determination of clonotypes and clonotype profiles from sequence data, and achieve the effect of efficient sequence comparison
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
TCRβ Repertoire Analysis: Amplification and Sequencing Strategy
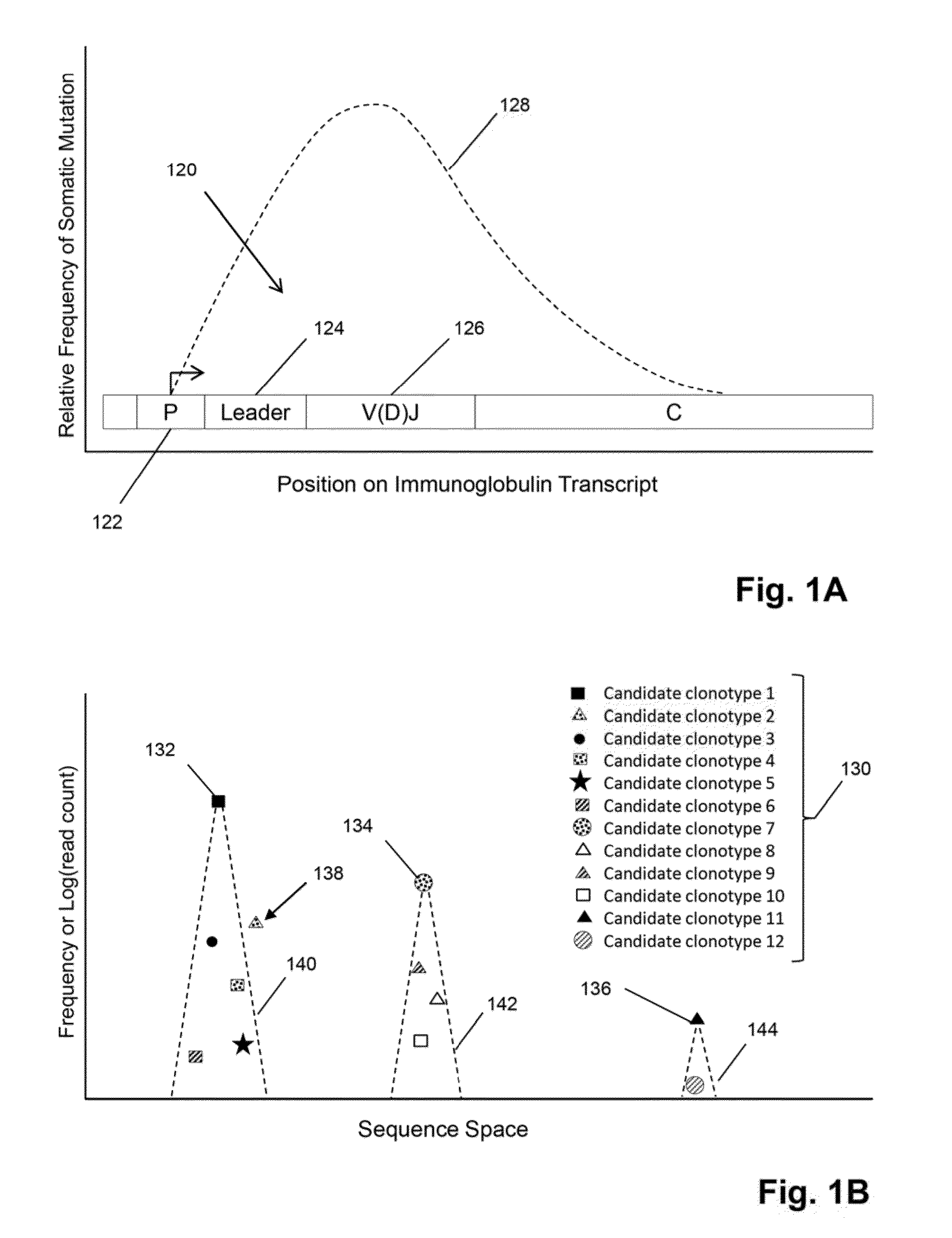
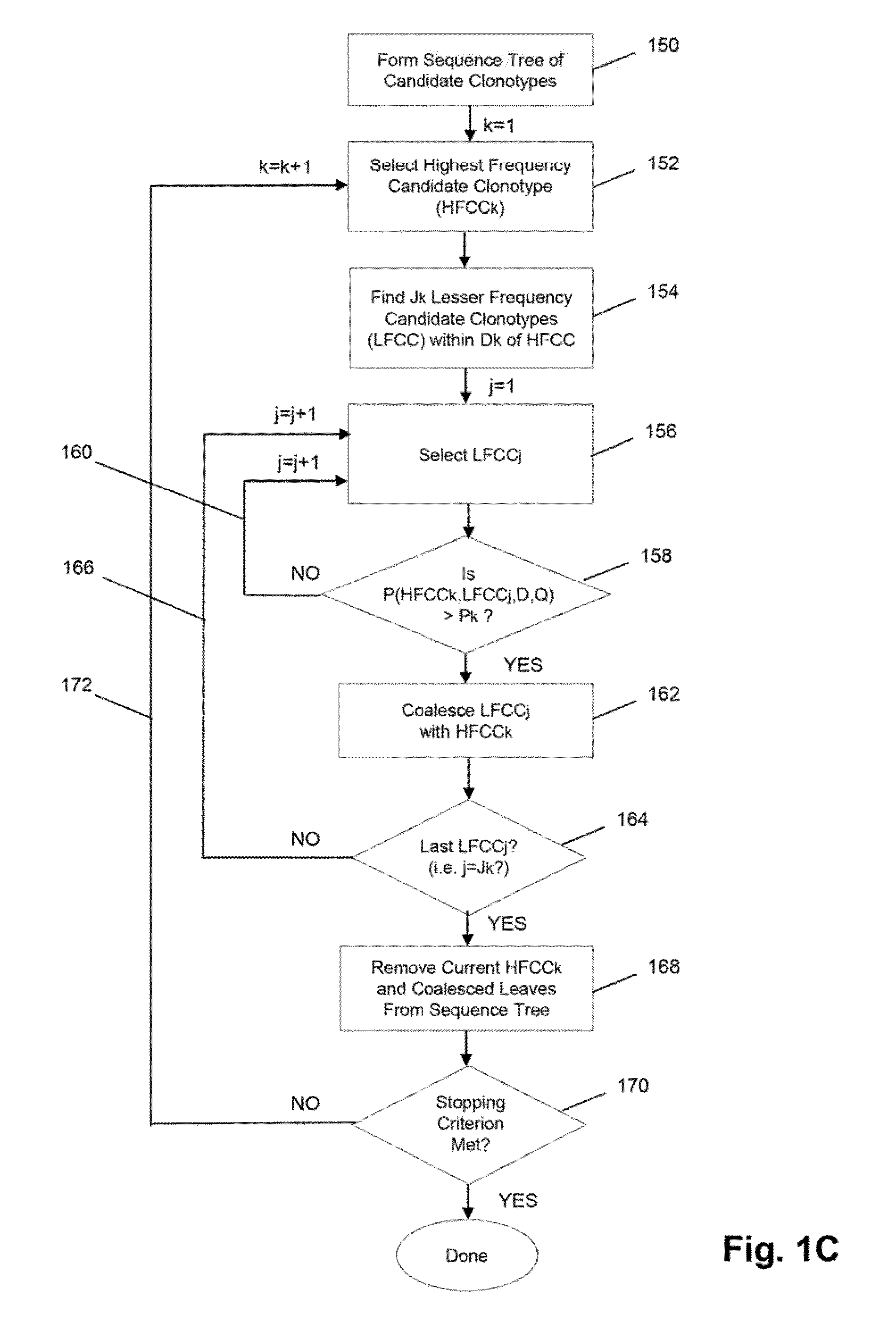
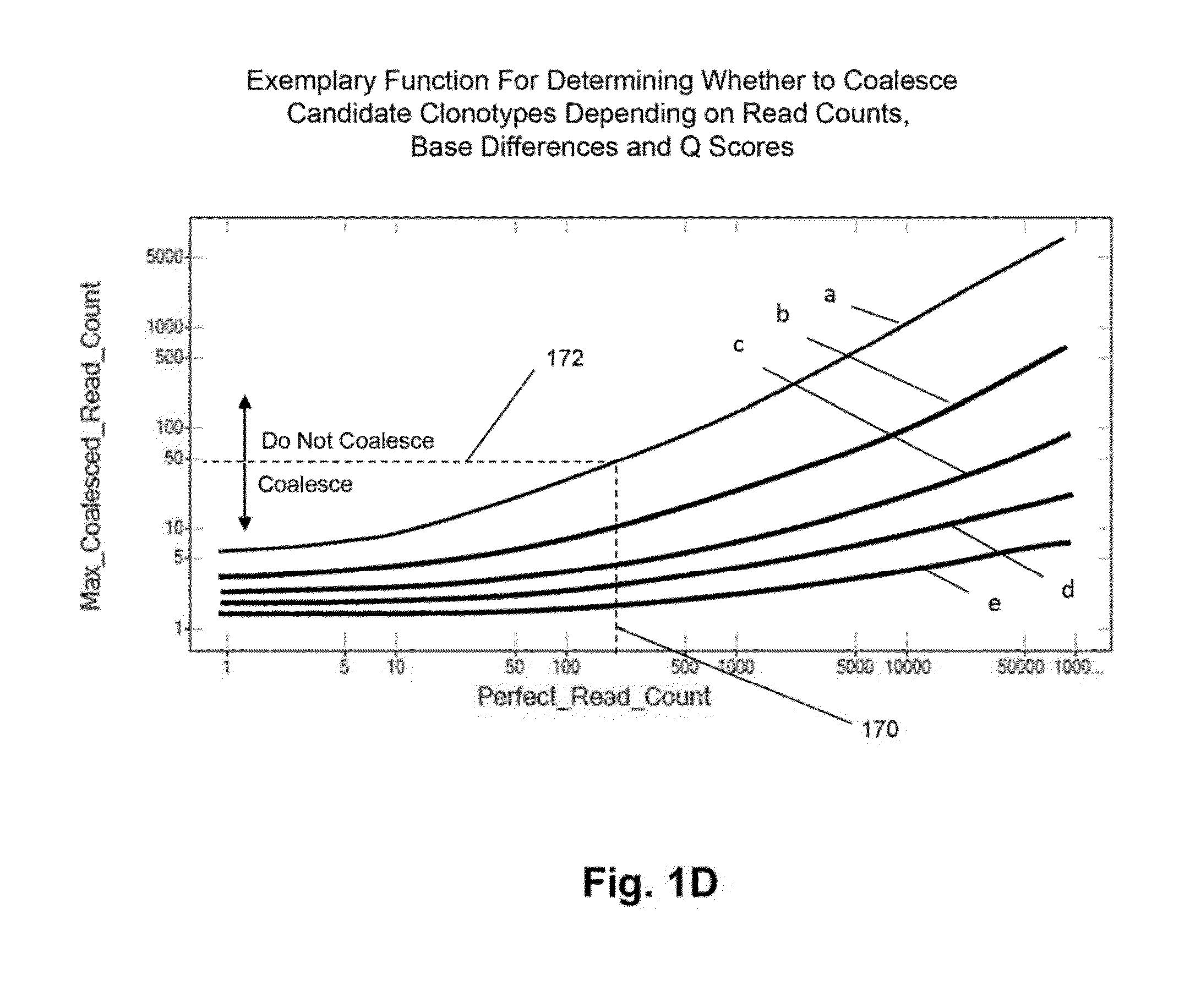
[0069]In this example, TCRβ chains are analyzed. The analysis includes amplification, sequencing, and analyzing the TCRβ sequences. One primer is complementary to a common sequence in Cβ1 and Cβ2, and there arc 34 V primers capable of amplifying all 48 V segments. Cβ1 or Cβ2 differ from each other at position 10 and 14 from the J / C junction. The primer for Cβ1 and Cβ2 ends at position 16 by and has no preference for Cβ1 or Cβ2. The 34 V primers are modified from an original set of primers disclosed in Van Dongen et al, U.S. patent publication 2006 / 0234234, which is incorporated herein by reference. The modified primers arc disclosed in Faham et al, U.S. patent publication 2010 / 0151471, which is also incorporated herein by reference.
[0070]The Illumina Genome Analyzer is used to sequence the amplicon produced by the above primers. A two-stage amplification is performed on messenger RNA transcripts (200), as illustrated in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com