Human antibody against aggrecanase-type adamts species for therapeutics of aggrecanase-related diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
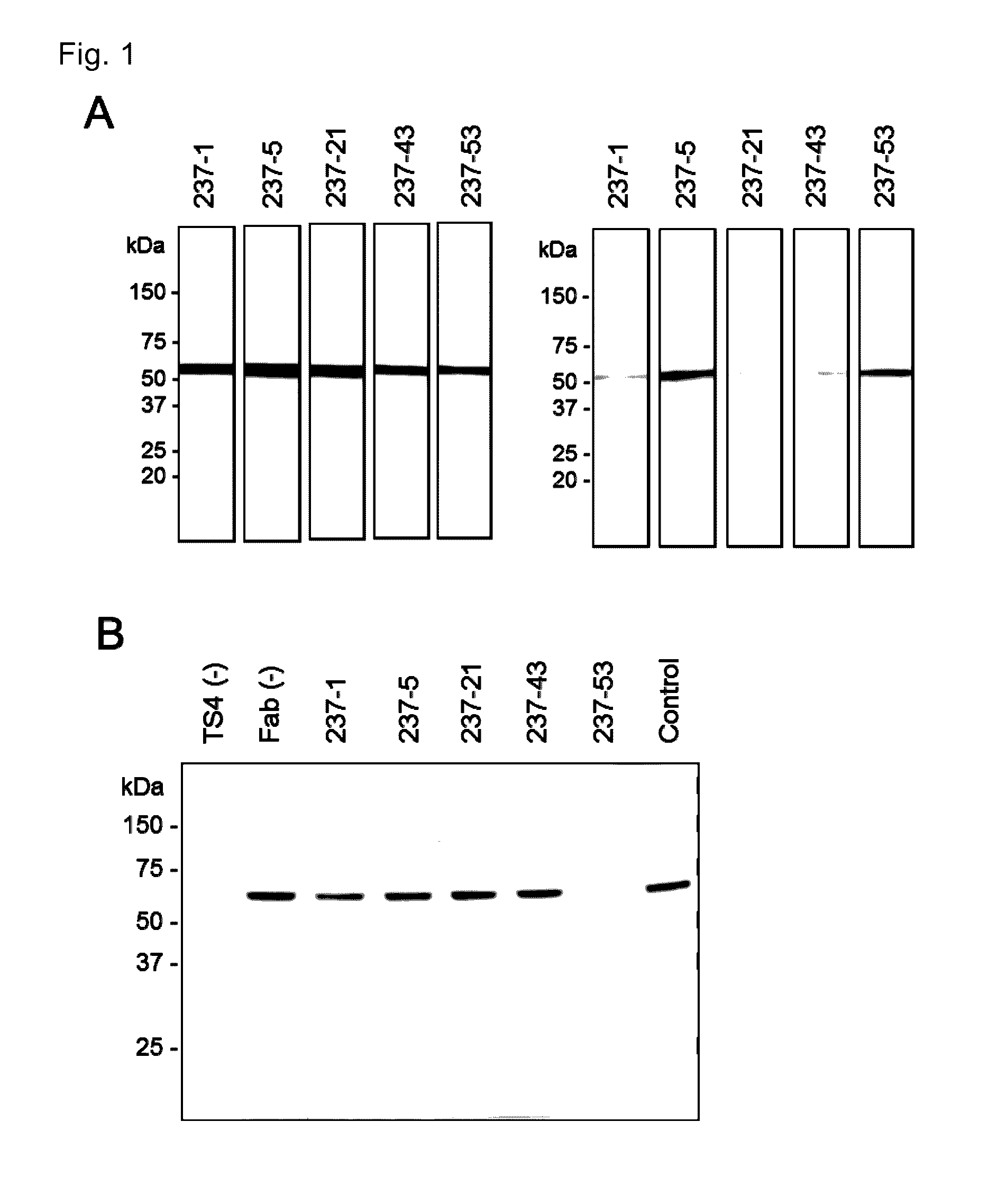
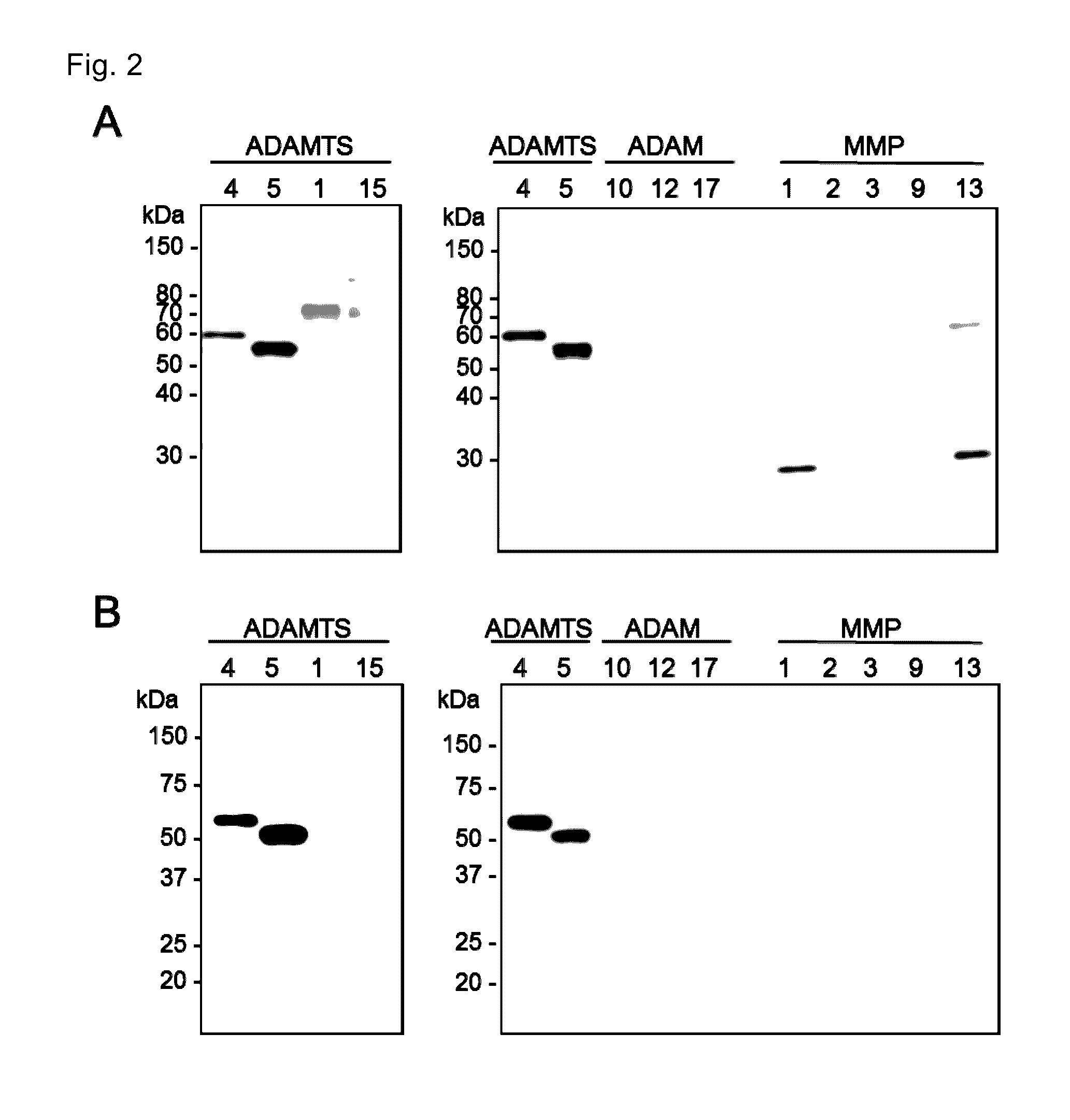
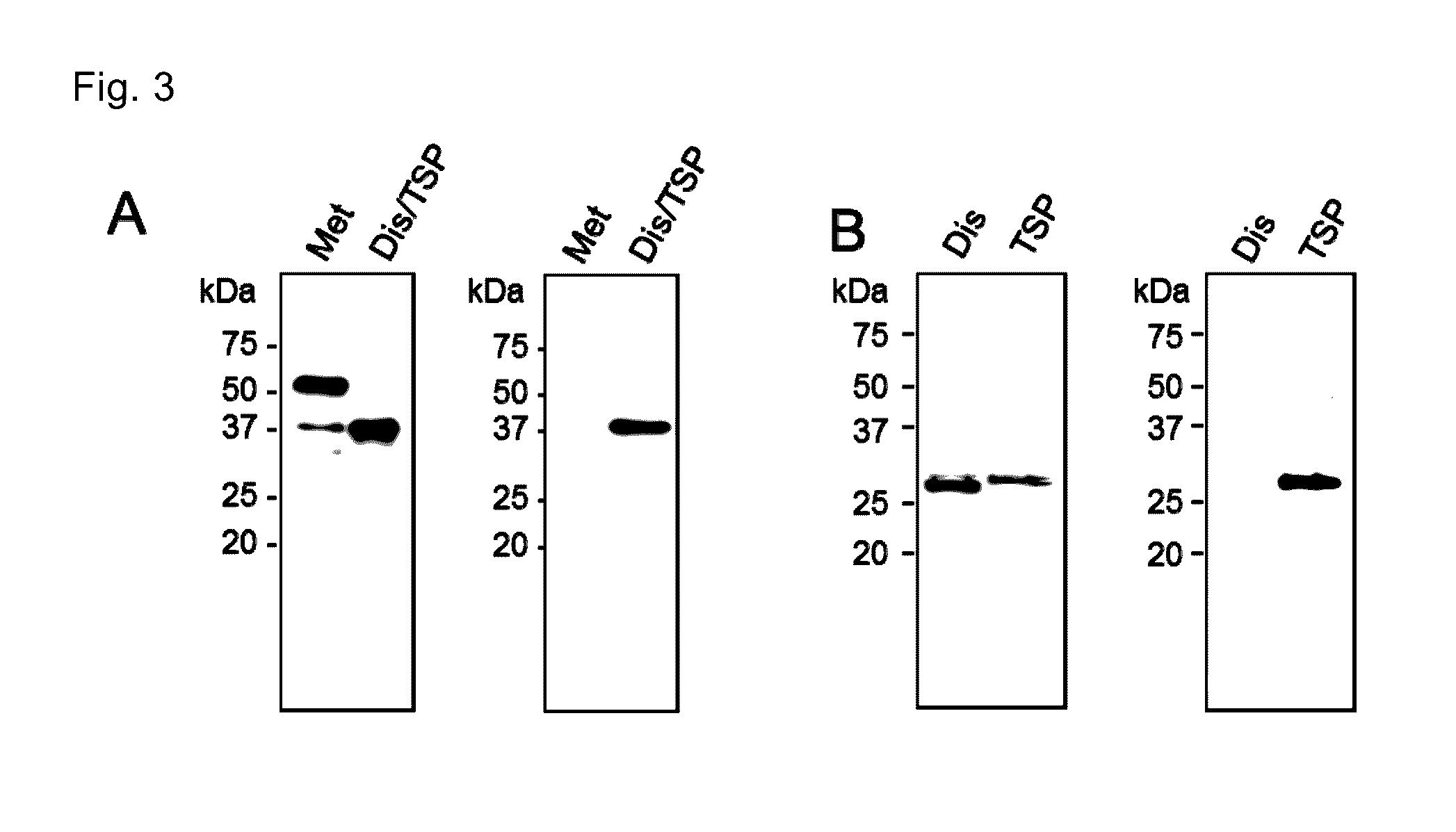
[0085]The present invention is explained in more detail in the following by referring to Examples, which are not to be construed as limitative. Various gene manipulations in the Examples followed the method described in Molecular cloning third. ed. (Cold Spring Harbor Lab. Press, 2001).
Materials and Methods
Phage Display Library Panning and Fab Generation
[0086]Production of monoclonal Fab antibodies specific to ADAMTS4 and ADAMTS5 were generated using the Human Combinatorial Antibody Library (HuCAL; MorphoSys AG, Martinried, Germany). Recombinant ADAMTS4 and ADAMTS5 (R&D Systems Inc., Minneapolis, Minn.) were biotinylated and incubated with HuCAL. Bound Fab expressing phages were enriched in three consecutive panning rounds. The pool of Fab genes was isolated from phagemids and inserted into Escherichia coli expression vectors that lead to functional periplasmic expression of Fab equipped with Strep-tag II. After transformation, individual colonies were picked up and grown in microti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com