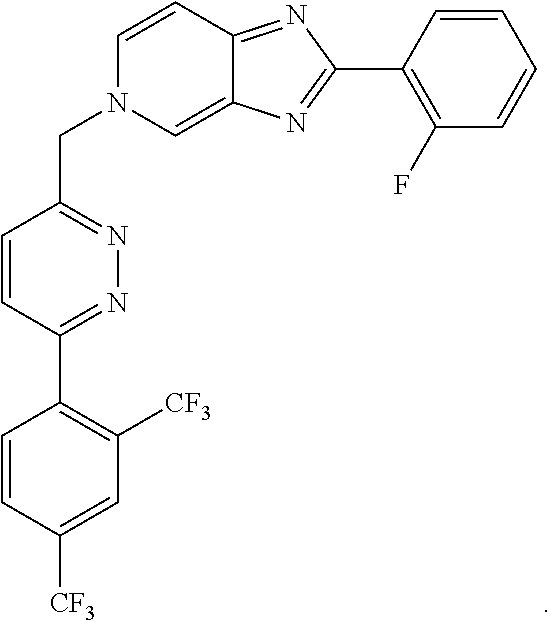
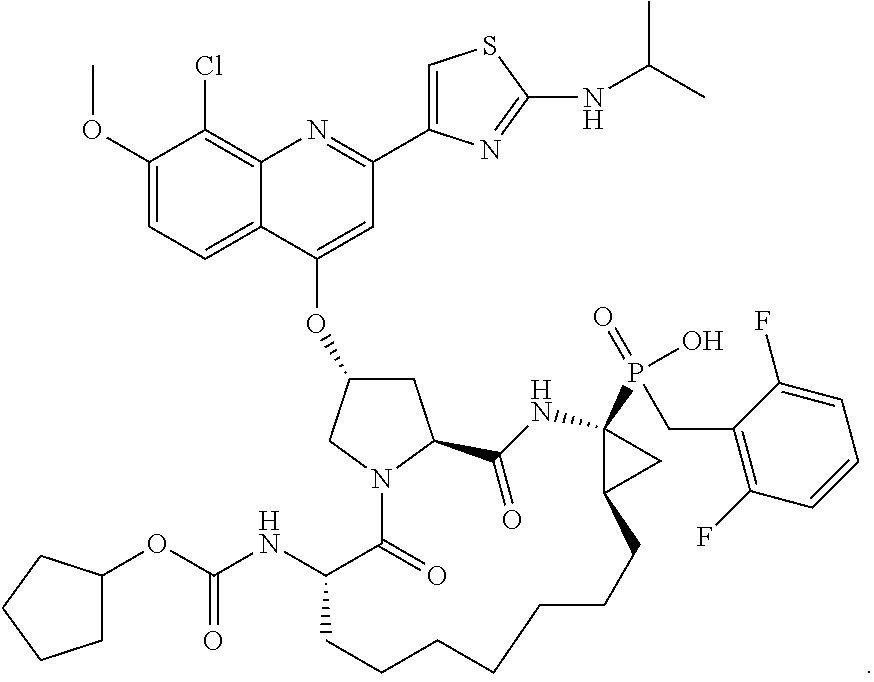
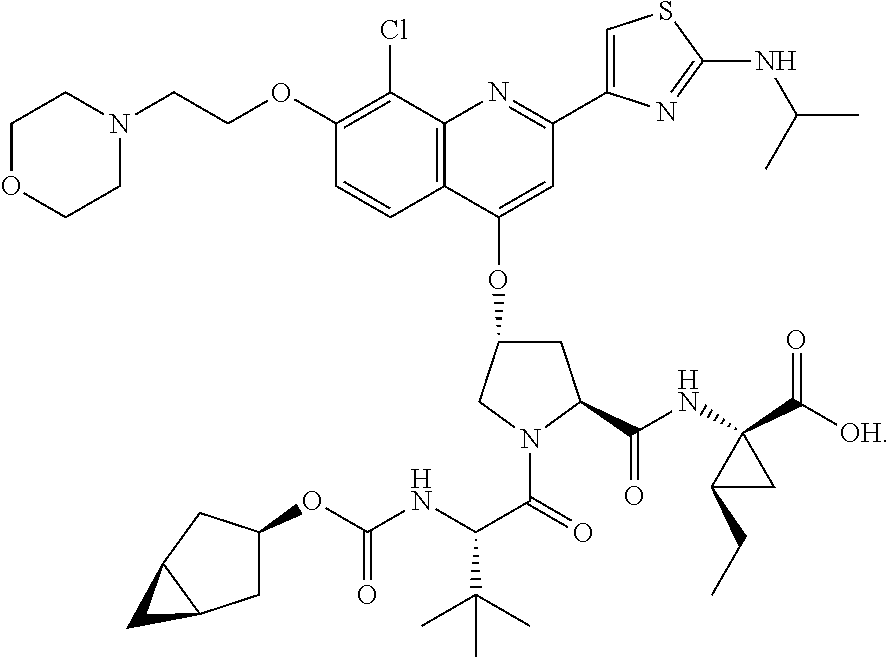
Methods for treating hcv
a technology for hepatitis c virus and therapeutic molecules, applied in the field of methods for treating hcv, can solve the problems of inability to start therapy, poor tolerance of treatment with peg-ifn+rbv, and many patients' refusal to start therapy, so as to reduce the potential for viral rebound, and shorten the less complicated dosing schedule
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of Compound 2
[0271]
[0272]Phosphinate ester 206 (23.7 g, 24.05 mmol) was dissolved in CH3CN (240 mL) and cooled to 0° C. lodotrimethylsilane (17.4 mL, 122.3 mmol) was added at a fast drop-wise pace followed by, after 10 min, 2,6-lutidine (17.0 mL, 146.4 mmol). The reaction mixture was slowly warmed to room temperature and stirred for 1 h then cooled back down to 0° C. and 2,6-lutidine (11.1 mL, 95.6 mmol) followed by MeOH (24 mL) were added. The solution was concentrated in vacuo and the crude residue was purified by HPLC to afford 12.68 g of Compound 2 in 55% yield. 1H NMR (300 MHz, CDCl3) δ 8.35 (d, J=9.3 Hz, 1H), 8.28 (s, 1H), 7.85 (s, 1H), 7.64 (d, J=9.6 Hz, 1H), 7.35-7.22 (m, 1H), 7.02-6.89 (m, 2H), 5.85 (bs, 1H), 4.82-4.71 (m, 2H), 4.33 (bs, 1H), 4.28-3.99 (m, 3H), 4.16 (s, 3H), 3.57-3.28 (m, 2H), 2.90-2.78 m, 1H), 2.63-2.50 (m, 1H), 2.08-1.91 (m, 1H), 1.91-170 (m, 2H), 1.70-1.13 (m, 22H), 1.37 (d, J=6.9 Hz, 6H); 31P NMR (121.4 MHz, CD3OD) δ 42.4; LCMS (M+1): 957.35...
example 3
Preparation of Compound 3
[0293]
[0294]Compound 315 (12 g, 13 mmol) was dissolved in THF (200 ml), LiOH (11 g, 260 mmol) in H2O (200 ml) was added, followed by MeOH (200 ml). The mixture was kept stirring at room temperature for 20 hours. Upon completion of the reaction, 4 N HCl in H2O was added to adjust pH to 7 at 0° C. The mixture was extracted with EtOAc (2×400 ml). The combined organic layer was washed with brine, dried (Na2SO4) and concentrated in vacuo to give compound 3 as a yellow solid (11 g, 93%). LC / MS=911.52 (M++1). 1H NMR (300 MHz, CD3OD) δ 7.95 (d, 1H), 7.90 (s, 1H), 7.48 (s, 1H), 7.31 (d, 1H), 5.42 (s, 1H), 4.37 (dd, 1H), 4.20 (m, 2H), 3.83-3.56 (m, 7H), 3.50 (m, 2H), 3.39 (m, 2H), 2.45 (m, 1H), 2.27 (m, 1H), 1.62 (m, 2H), 1.50 (m, 1H), 1.33 (m, 2H), 1.18 (m, 1H), 1.05 (m, 8H), 0.90 (m, 3H), 0.76 (m, 11H), 0.14-0.04 (m, 2H)
[0295]The intermediate compound 315 was prepared as follows.
a. Preparation of Compound 301
[0296]To a dry, argon purged three-neck round bottom flask...
example 4
Preparation of Compound 4
[0311]
[0312]Diastereomeric mixture 414 was dissolved in heptane and isopropanol (70%:30%, 230 mg in 4.5 mL of the mixed solvents) and subjected to chiral column separation under the following conditions:
[0313]Column: Chiralcel OD-H, 2×25 cm
[0314]Solvent system: 70% heptane and 30% isopropanol
[0315]Flow rate: 6 mL / min.
[0316]Loading volume per run: 2.5 mL
[0317]Compound 4 had a retention time of 20 minutes. 1H NMR (300 MHz, CDCl3): δ 8.00 (s, 1H), 7.1-7.3 (m, 5H), 6.83 (d, 1H), 6.71 (d, 1H), 6.09 (brs, 2H), 5.95 (s, 1H), 5.04 (m, 2H), 4.67 (q, 1H), 4.35-4.52 (m, 2H), 4.00 (m, 2H), 2.74 (m, 1H), 1.40 (d, 3H), 1.2-1.3 (12H), 0.98 (s, 3H). 31P NMR (121.4 MHz, CDCl3): δ 2.72 (s). Compound 4 was subsequently recrystallized from MTBE for x-ray quality crystals.
[0318]Compound 4a had a retention time 50 min. 1H NMR (300 MHz, CDCl3): δ 7.98 (s, 1H), 7.1-7.3 (m, 5H), 6.83 (d, 1H), 6.73 (d, 1H), 6.02 (brs, 2H), 5.95 (s, 1H), 5.08 (d, 1H), 5.00 (m, 1H), 4.68 (q, 1H), 4.38-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com