Use of 1,3-propanedisulfonic acid or pharmaceutically acceptable salts thereof for the treatment of sarcoidosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
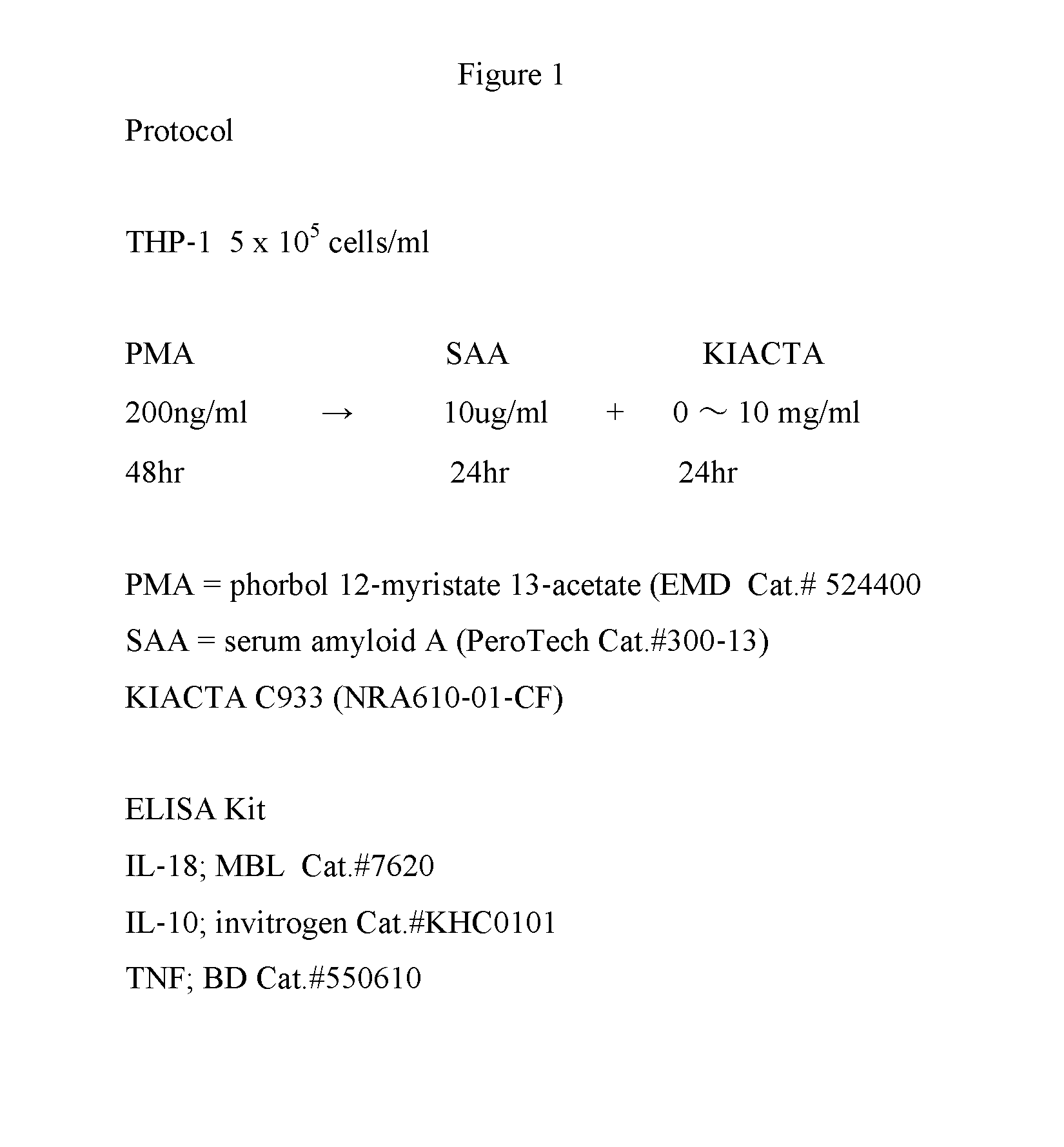
[0063]THP-1 cells were subjected to PMA (phorbol 12-myristate 13-acetate (EMD Cat.#524400)), SAA (serum amyloid A (PeroTech Cat.#300-13)), and 1,3-propanedisulfonic acid (KIACTA C933 (NRA610-01-CF)). The protocol is summarized in FIG. 1. The SAA treated cells were used as a positive control, with the SAA increasing the inflammatory mediators, TNF, IL-18 and IL-10. 1,3-propanedisulfonic acid is added to the cell cultures and the effect on TNF, IL-18 and IL-10 levels is measured.
example 2
[0064]In the protocol described in Example 1, 1,3-propanedisulfonic acid at a concentration of 5 mg / ml and above inhibits SAA-induced production of the inflammatory mediator, IL-18 by the THP-1 cells.
[0065]In the protocol described in Example 1, 1,3-propanedisulfonic acid at a concentration of 2.5 mg / ml and above inhibits SAA-induced production of the inflammatory mediator, IL-10 by the THP-1 cells.
[0066]In the protocol described in Example 1, 1,3-propanedisulfonic acid at a concentration of 1.25 mg / ml and above inhibits SAA-induced production of the inflammatory mediator, IL-TNF by the THP-1 cells.
example 3
[0067]A patient is diagnosed with sarcoidosis, and 1,3-propane disulfonic acid is administered to the patient. The treatment results in the reduction and / or alleviation of sarcoidosis symptoms in the patient.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap