Methods of Preparing T Cells for T Cell Therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0176]E1. A method for delaying or inhibiting T cell maturation or differentiation in vitro for a T cell therapy, comprising contacting one or more T cells from a subject in need of a T cell therapy with an AKT inhibitor and at least one of exogenous Interleukin-7 (IL-7) and exogenous Interleukin-15 (IL-15), wherein the resulting T cells exhibit delayed maturation or differentiation, and / or wherein the resulting T cells exhibit improved T cell function relative to a T cell function of a T cell cultured in the absence of an AKT inhibitor.
[0177]E2. A method for improving T cell function in vitro for a T cell therapy, comprising contacting one or more T cells from a subject in need of a T cell therapy with an AKT inhibitor and at least one of exogenous Interleukin-7 (IL-7) and exogenous Interleukin-15 (IL-15), wherein the resulting T cells exhibit an improved T cell function relative to a T cell function of a T cell cultured in the absence of an AKT inhibitor.
[0178]E3. The method of E1...
example 1
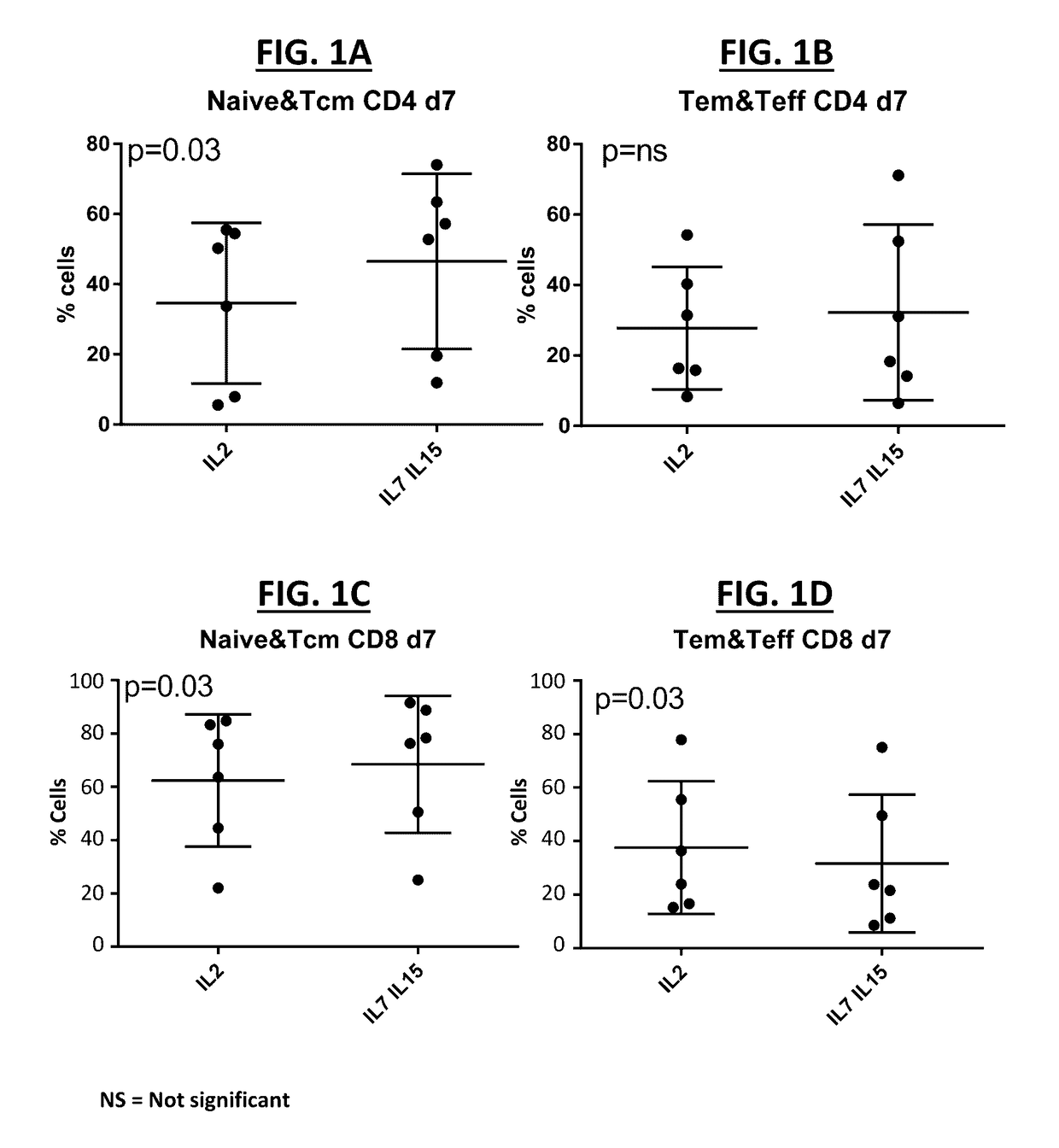
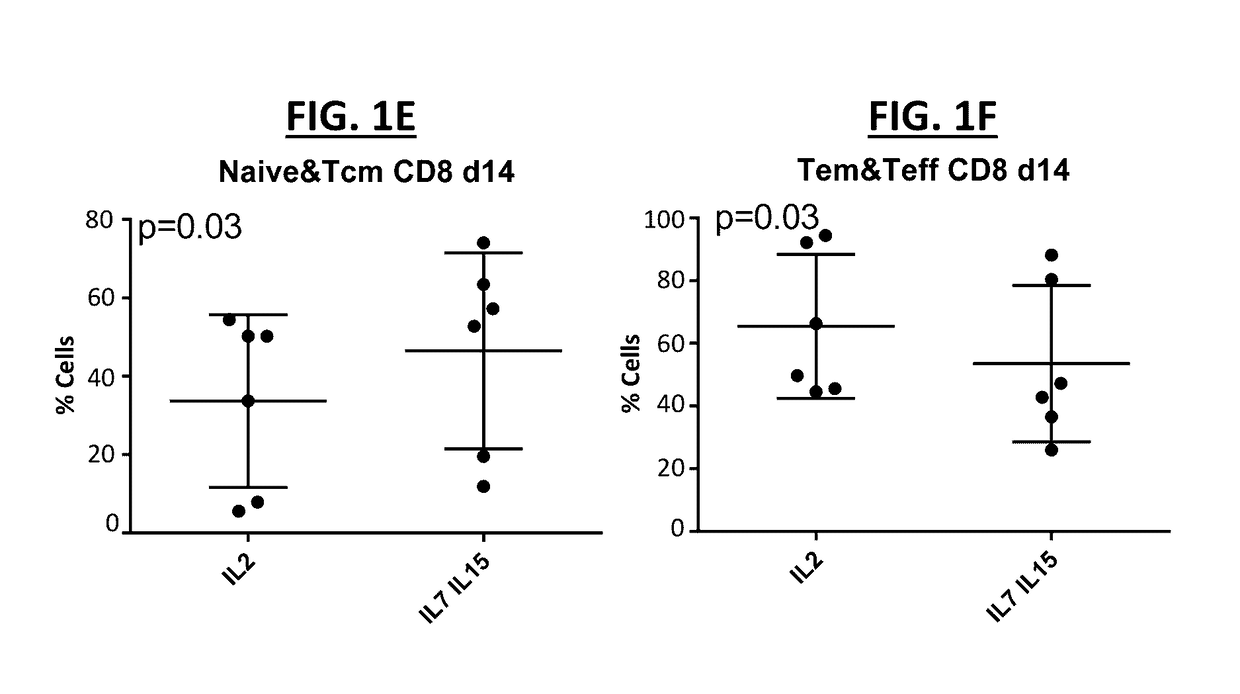
[0240]Donor T cells were cultured in the presence of IL-2, IL-7, IL-15, and / or an AKT inhibitor for 10 days, at equal plating concentrations. The T cell phenotype of the cultured T cells was determined for CD4+ T cells and CD8+ T cells cultured for 7 and 14 days in (i) IL-2 alone as compared to IL-7 and IL-15 and (ii) cultured in IL-7 and IL-15 as compared to IL-7, IL-15, and an AKT inhibitor (FIG. 1A-FIG. 1F). A trend towards more juvenile T-cells was observed when cells were grown in the presence of IL-7 and IL-15. In particular, a significantly higher (p=0.03, n=6) percent of naïve and Tcm cells CD4+ was observed in IL-7 / IL-15 treated cells as compared to IL-2 treated cells (FIG. 1A), whereas no difference was observed in the percent of more mature effector T cells (FIG. 1B). This effect was not maintained after long term culture in the CD4+ compartment (data now shown). A similar effect was observed in the CD8+ compartment, with a significantly higher (p=0.03, n=6) percent of na...
example 2
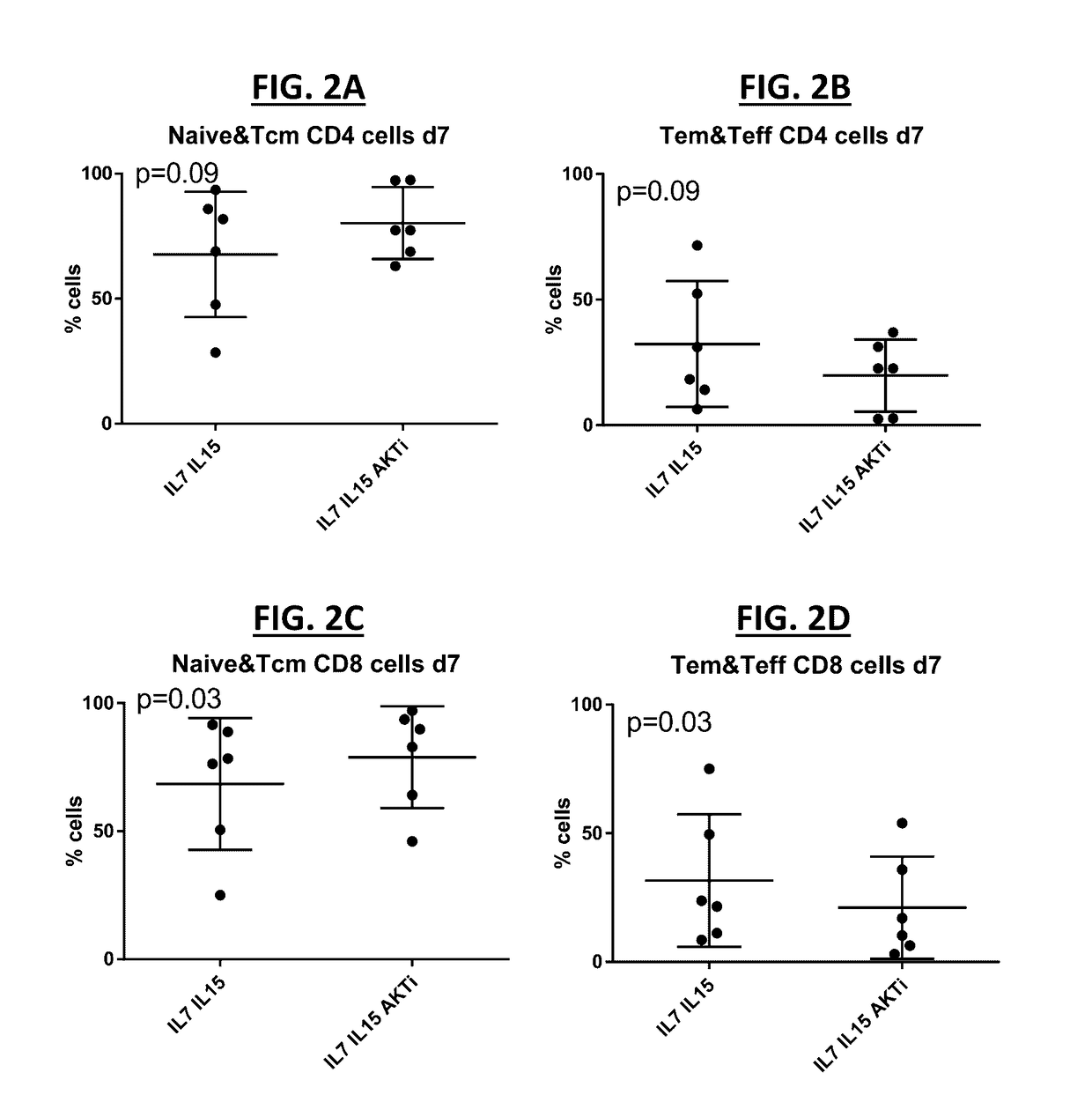
[0243]The effects of AKTi inhibitors on cell expansion were investigated under various conditions. First, the effect of AKTi culture conditions on various sources of donor cells was evaluated as follows. Apheresis products from four healthy donors were processed using high density centrifugation to obtain peripheral blood mononuclear cells (PBMCs) (FIGS. 4A-4D). Cells from four donors were counted and stimulated using OKT3 (a monoclonal antibody to CD3), and cultured in the presence of IL-2 (circles); IL-2 and AKTi (squares); IL-7 and IL-15 (triangles); or IL-7, IL-15, and AKTi (inverted triangles) for 7 to 10 days (FIGS. 4A-4D). Cell expansion was observed for each donor cell line under each culture condition, and AKTi had no negative impact on cell expansion (FIGS. 4A-4D).
[0244]Next, class I TCR transduced (HPV-E6) PBMCs were evaluated. Apheresis products from three healthy donors were again processed using high density centrifugation to obtain PBMC, counted, and stimulated using ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap