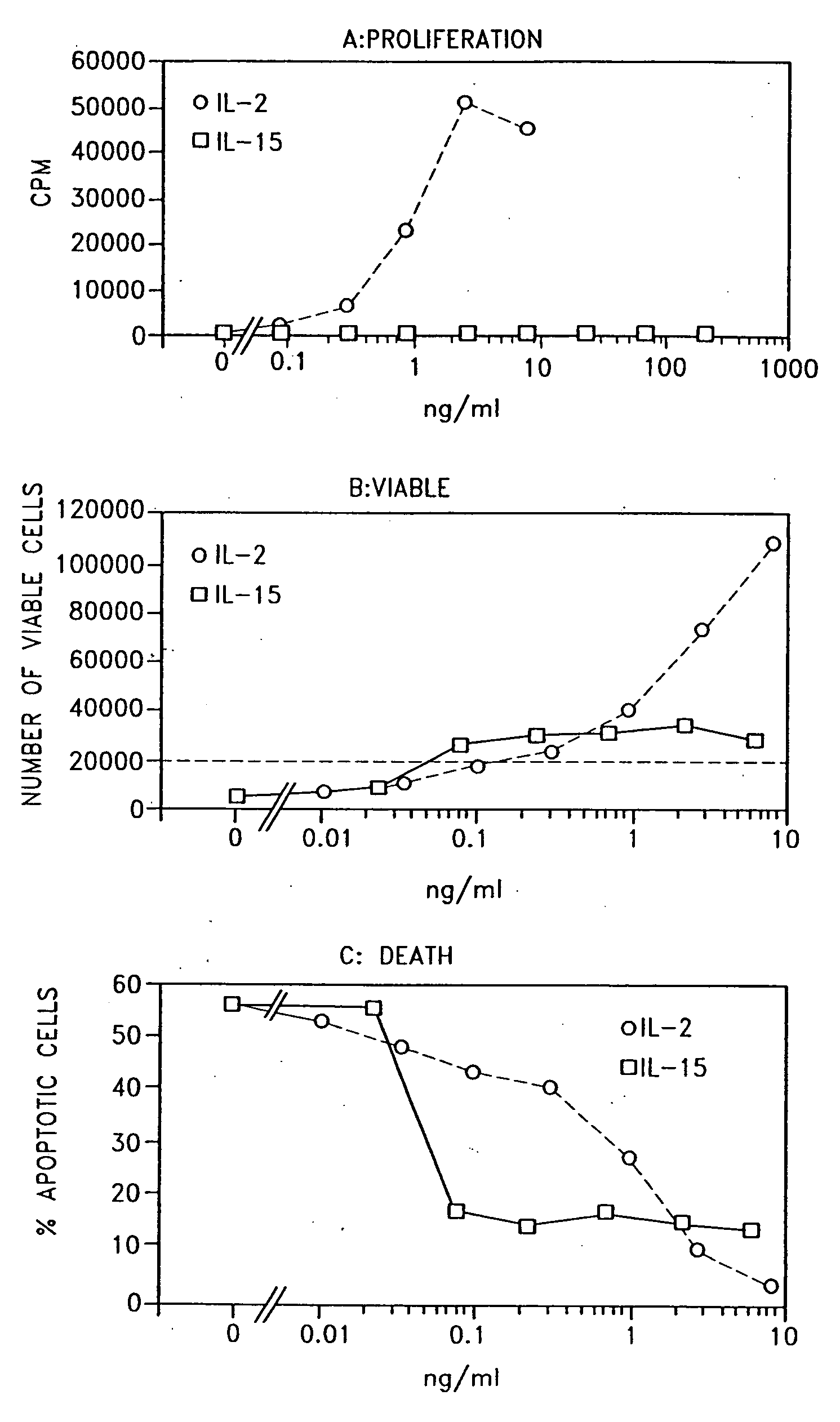
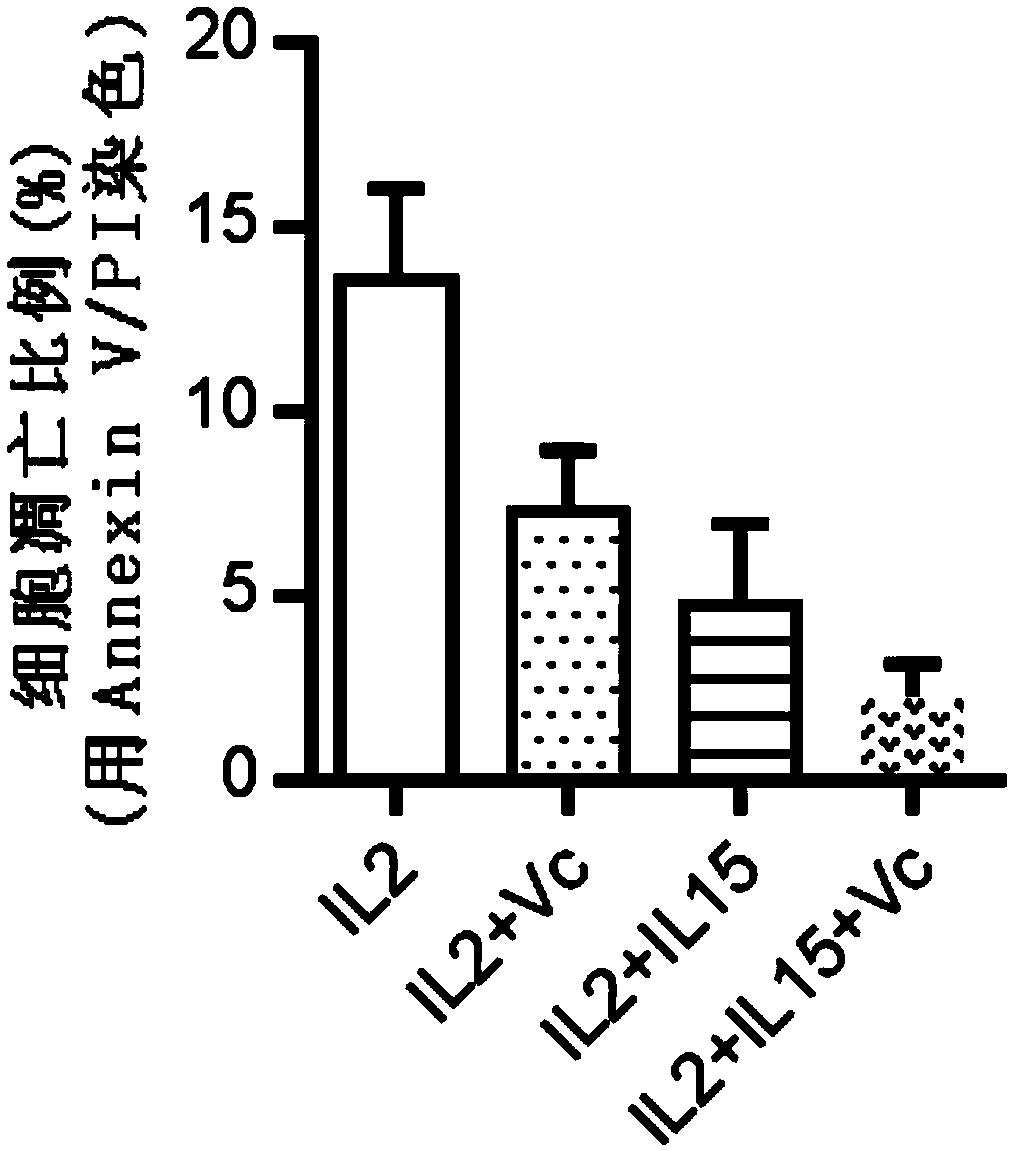Patents
Literature
86 results about "Interleukin 15" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
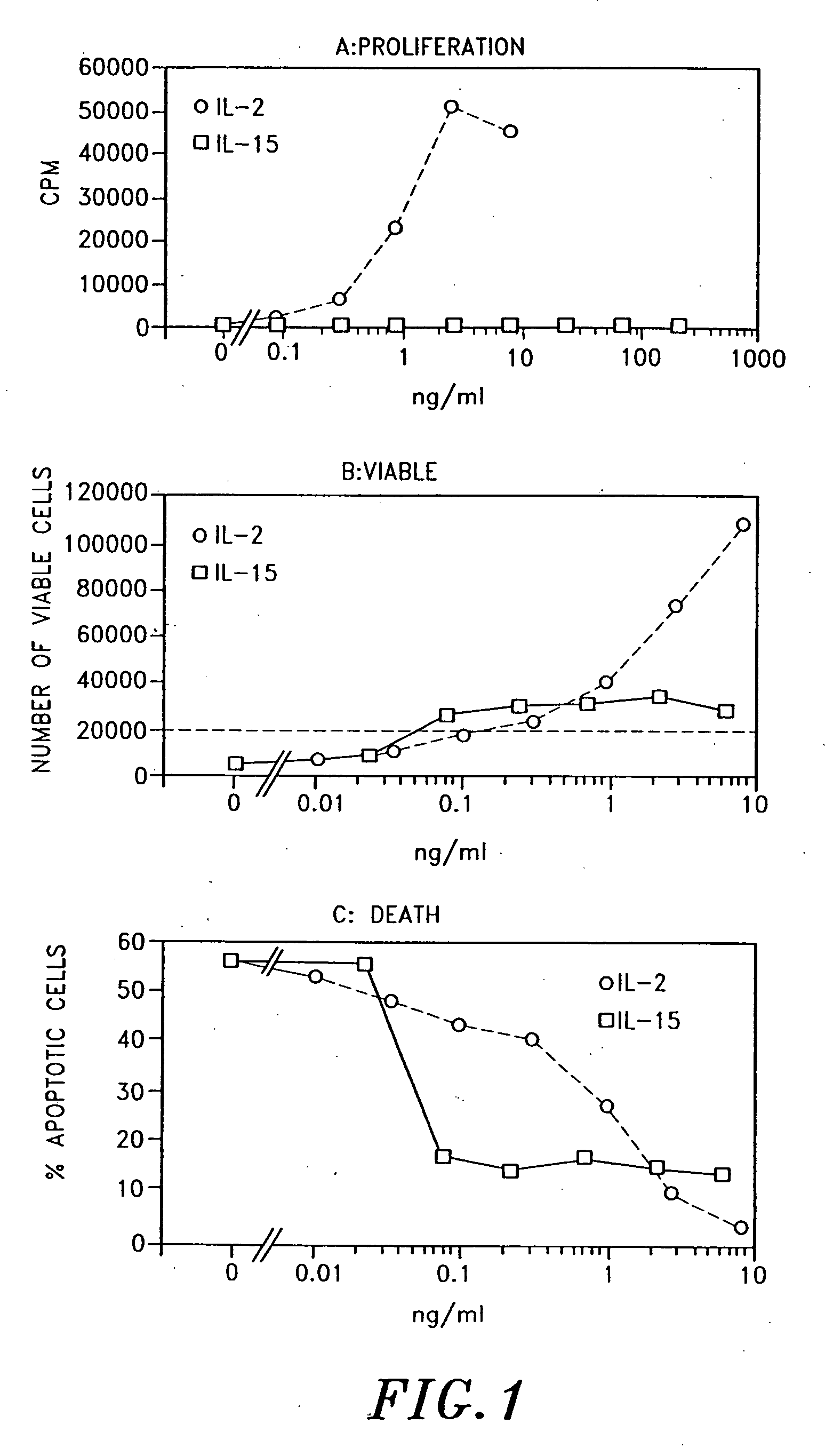
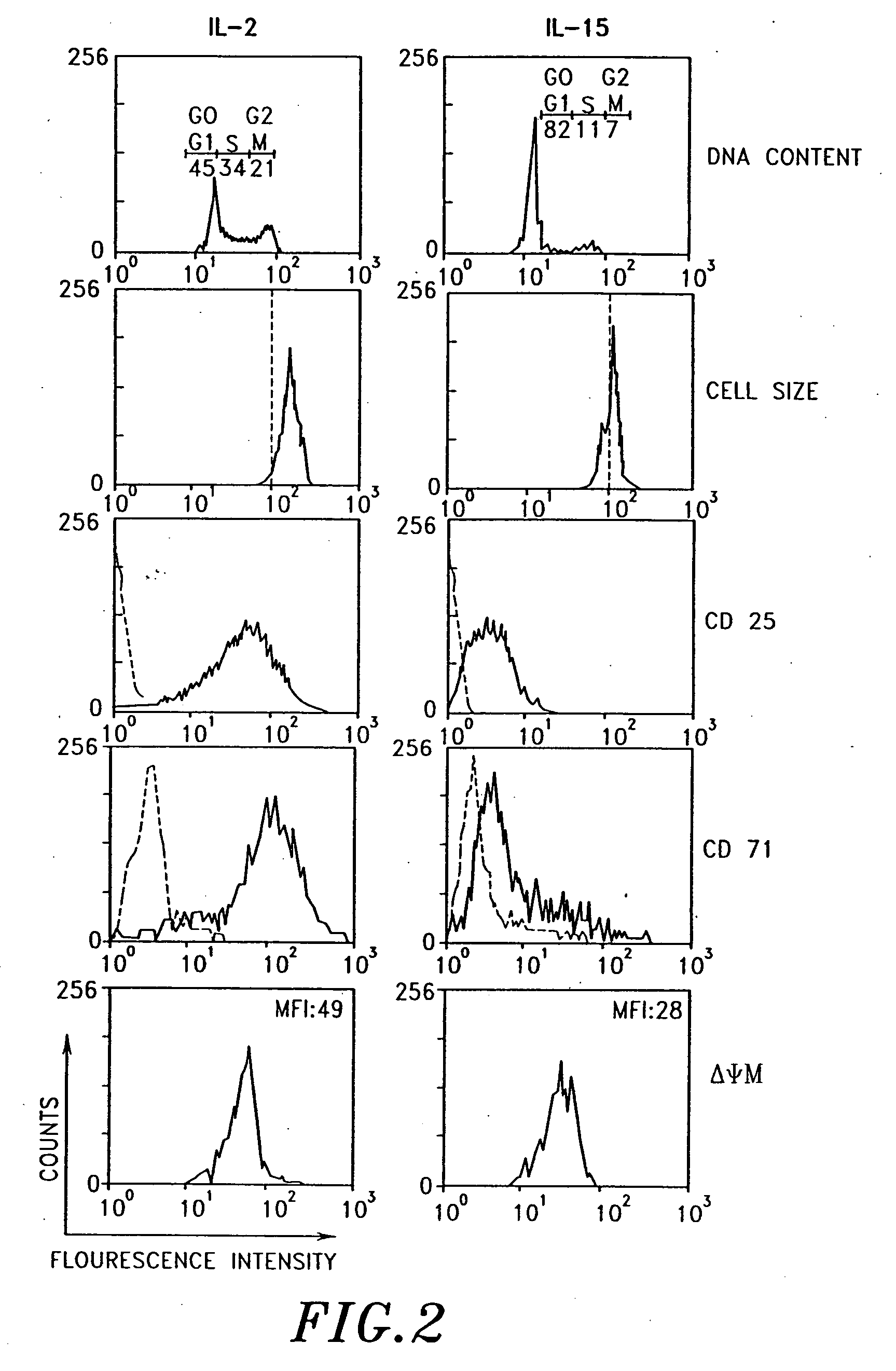
Interleukin-15 (IL-15) is a cytokine with structural similarity to Interleukin-2 (IL-2). Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain (gamma-C, CD132). IL-15 is secreted by mononuclear phagocytes (and some other cells) following infection by virus(es). This cytokine induces cell proliferation of natural killer cells; cells of the innate immune system whose principal role is to kill virally infected cells.
Expansion of lymphocytes with a cytokine composition for active cellular immunotherapy
PendingUS20170107490A1Increase stimulationImprove scalabilityPeptide/protein ingredientsBiological material analysisTissue sampleLymphocyte
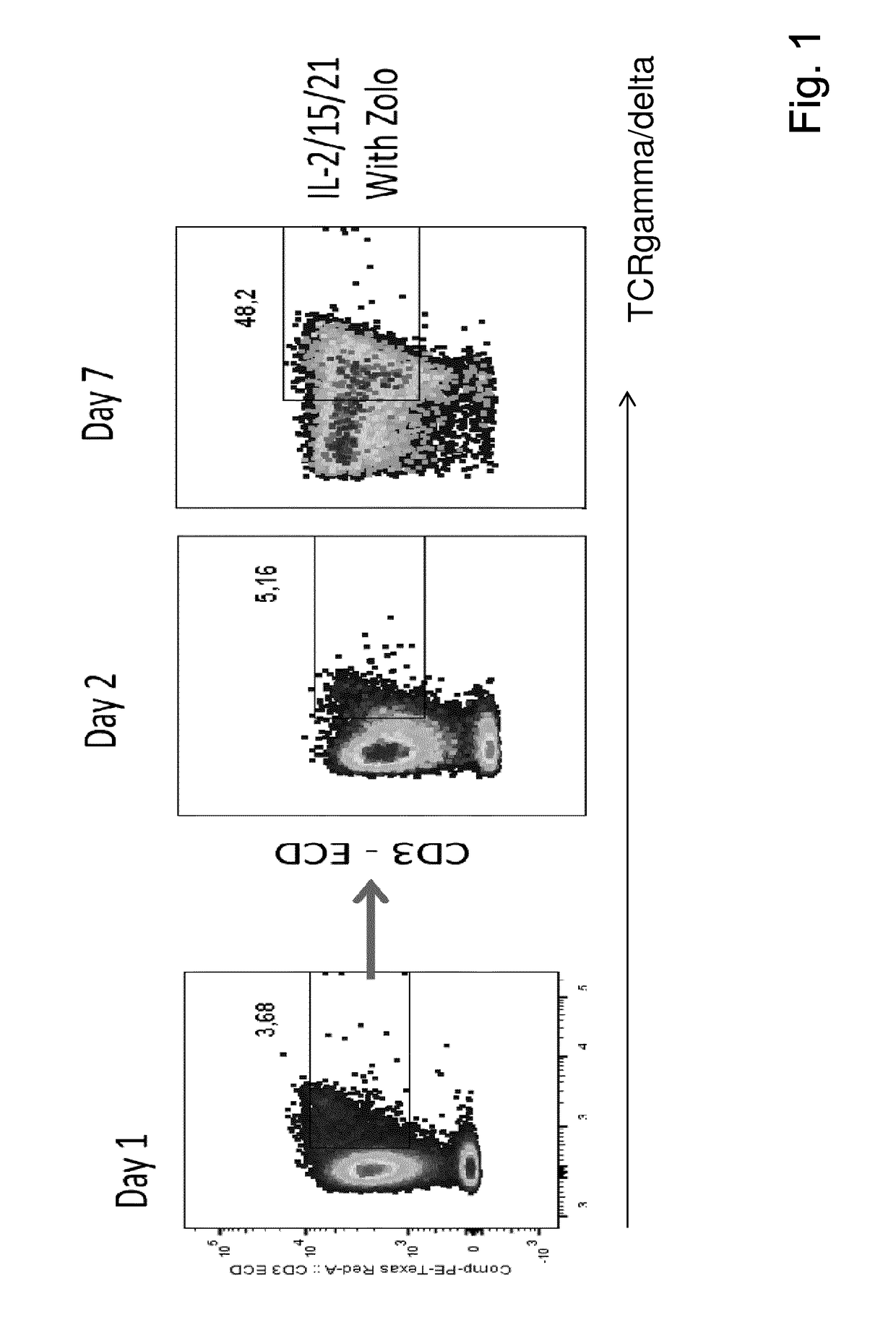
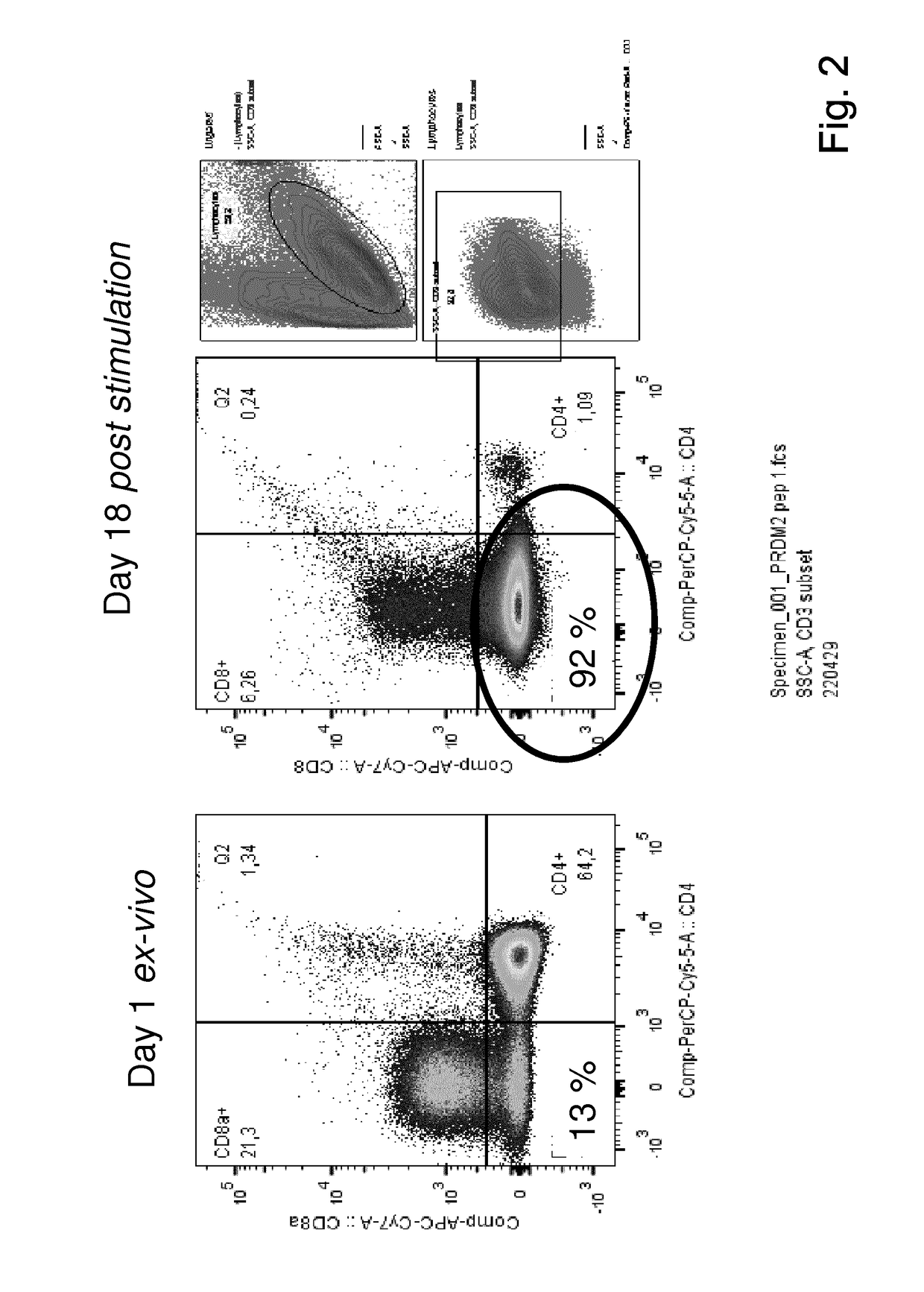
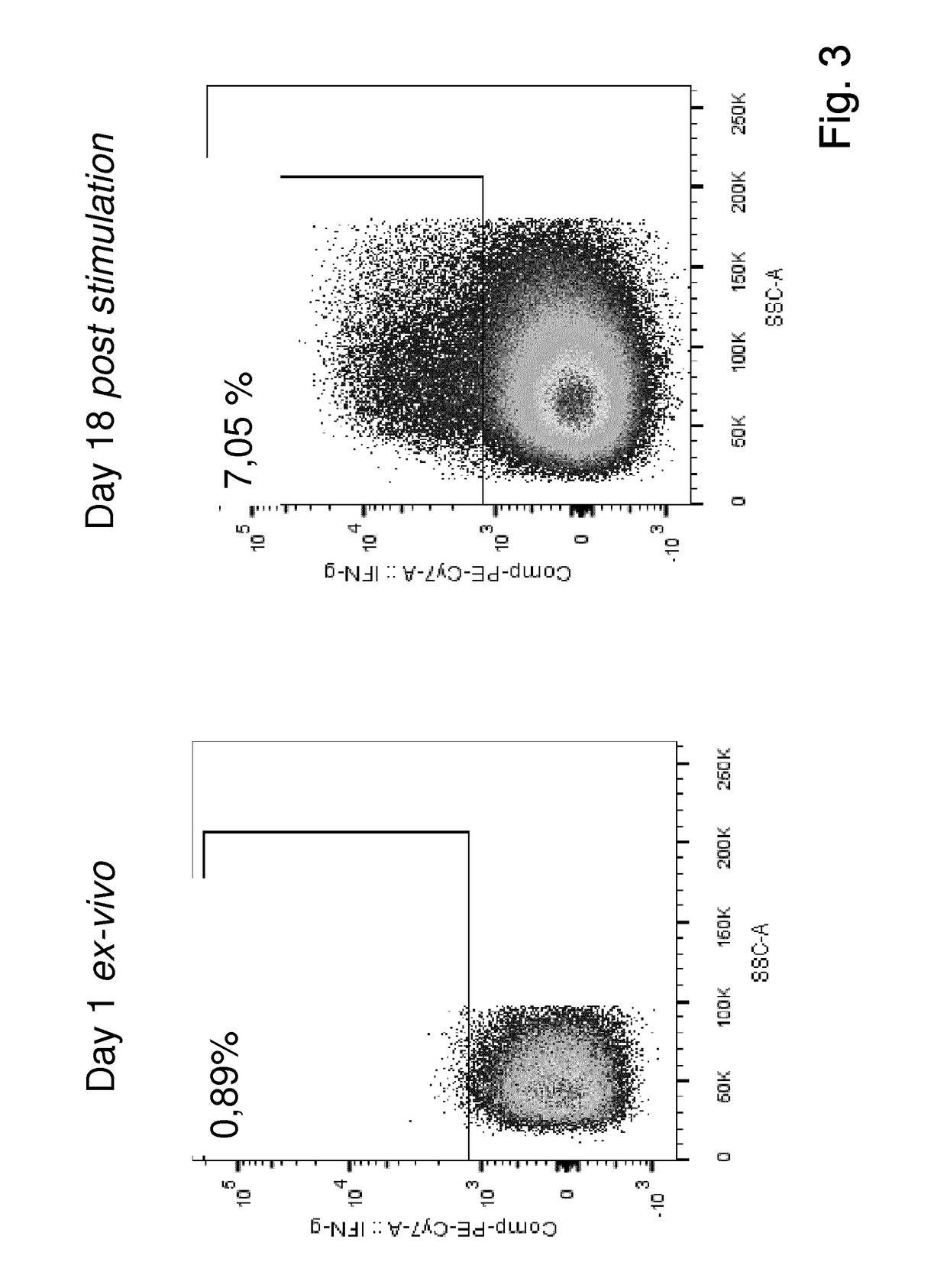
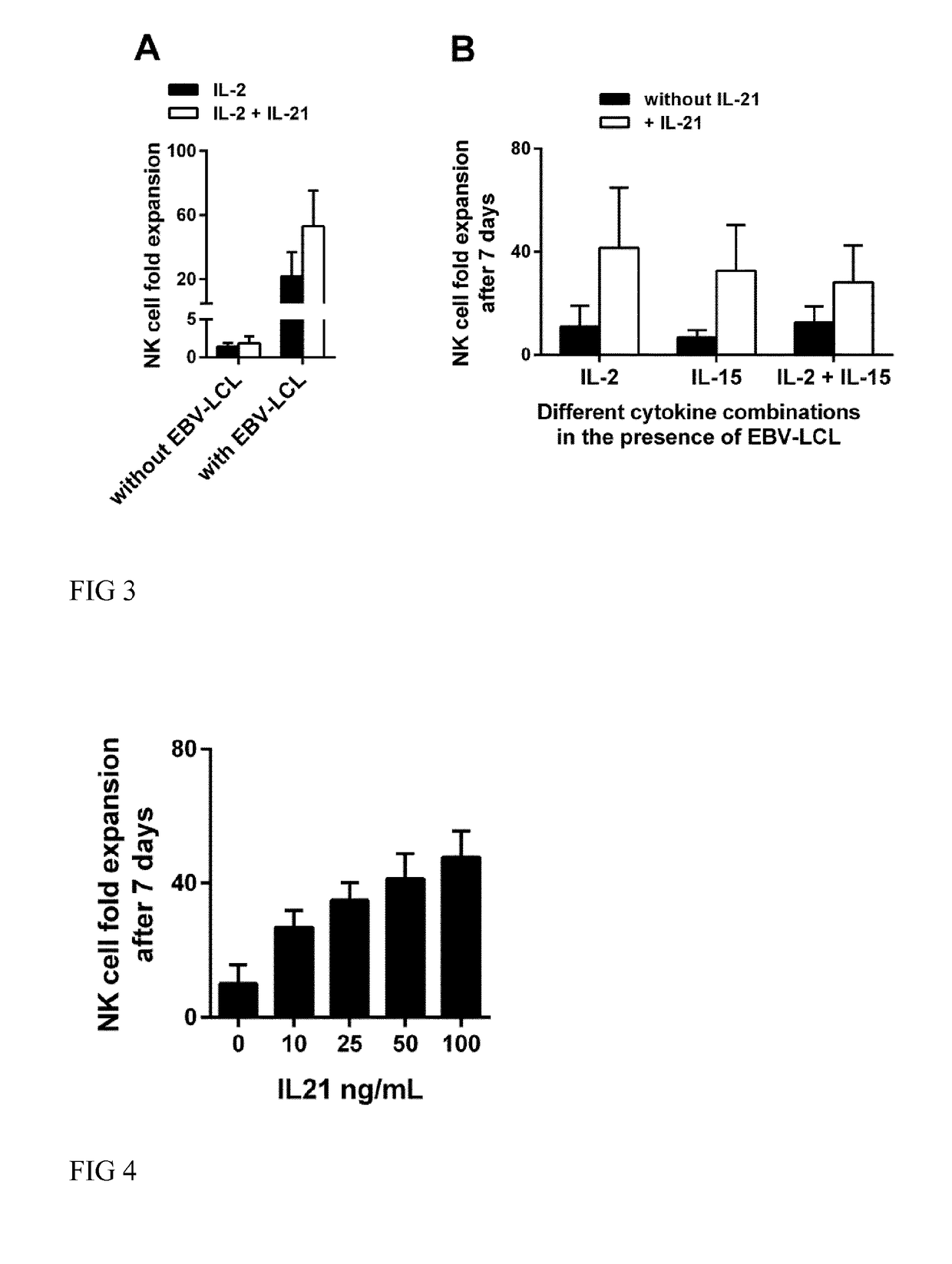
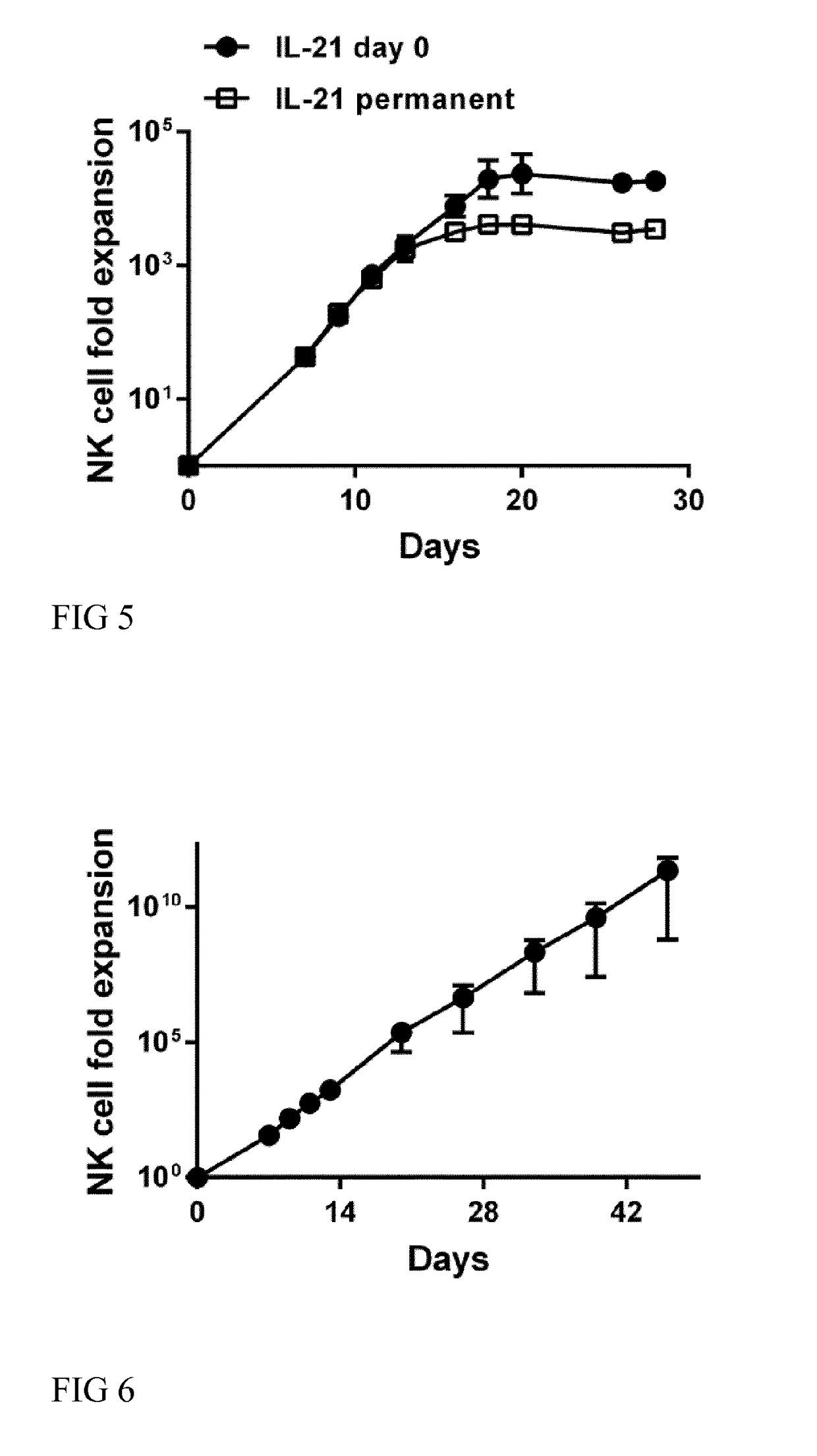
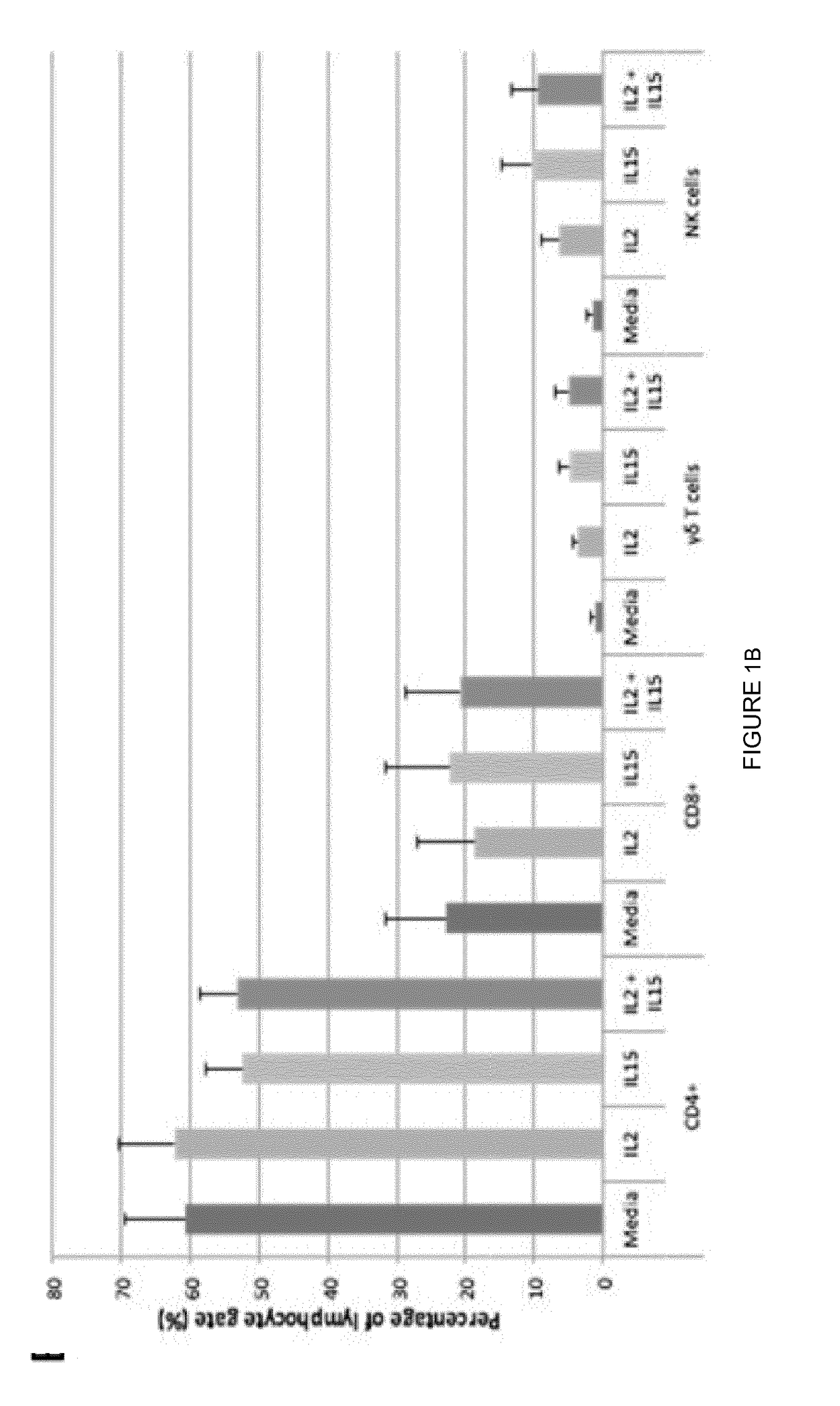
The present invention relates to a composition for expanding lymphocytes comprising at least two types of cytokines selected from interleukin 2 (IL-2), interleukin 15 (IL-15) and interleukin 21 (IL-21). It further relates to a Method of preparing a population of clinically relevant lymphocytes, comprising the steps of: obtaining a body sample from a mammal in particular a tissue sample or body liquid sample, comprising at least one lymphocyte and optionally separating the cells in the body sample, culturing the body sample in-vitro to expand and / or stimulate lymphocytes in the sample wherein the culturing comprises using IL-2, IL-15 and / or IL-21, and optionally determining the presence of clinically relevant lymphocyte in the cultured sample. The present invention also relates to an immunotherapy and the population of clinically relevant lymphocytes.
Owner:POLYBIOCEPT GMBH
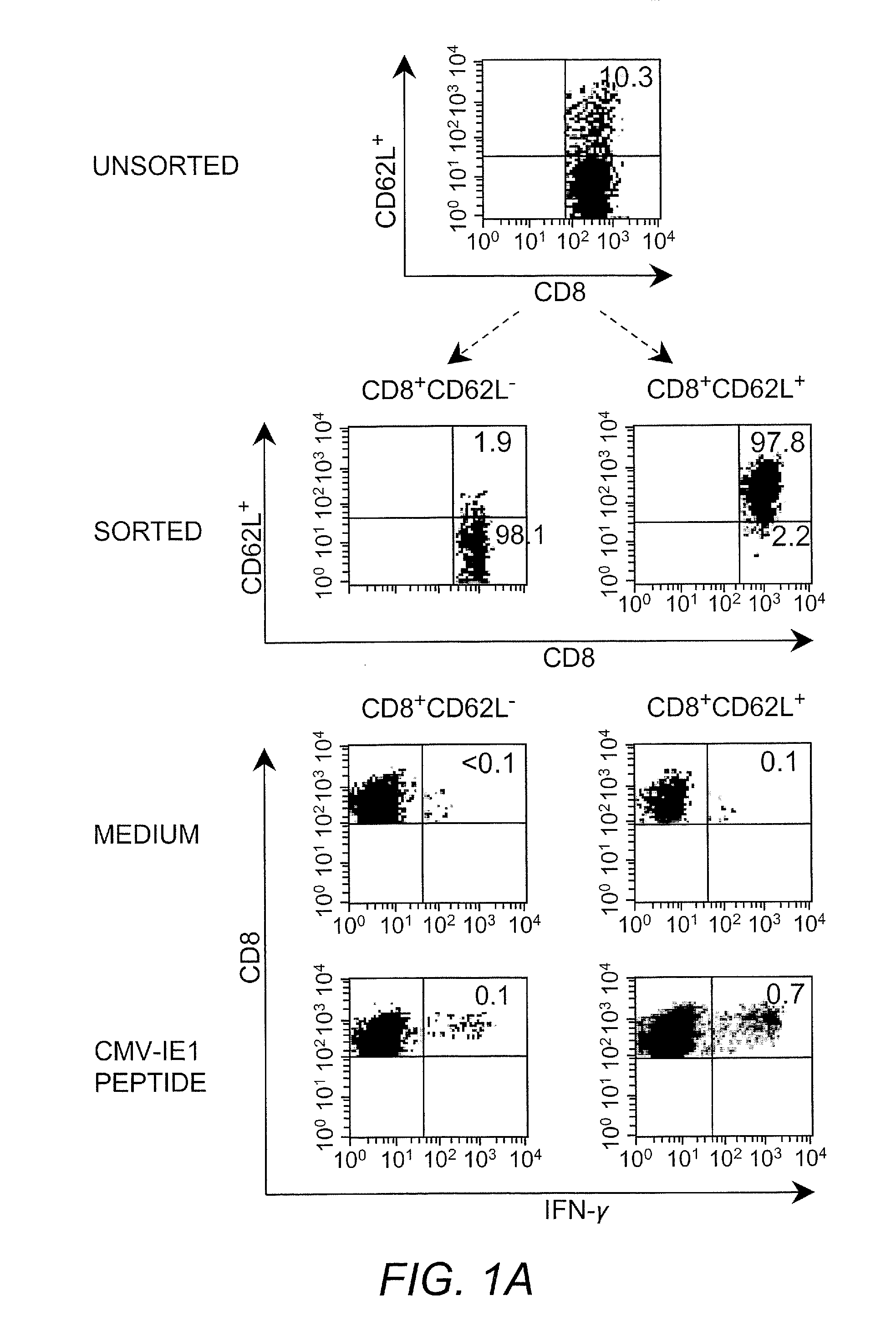
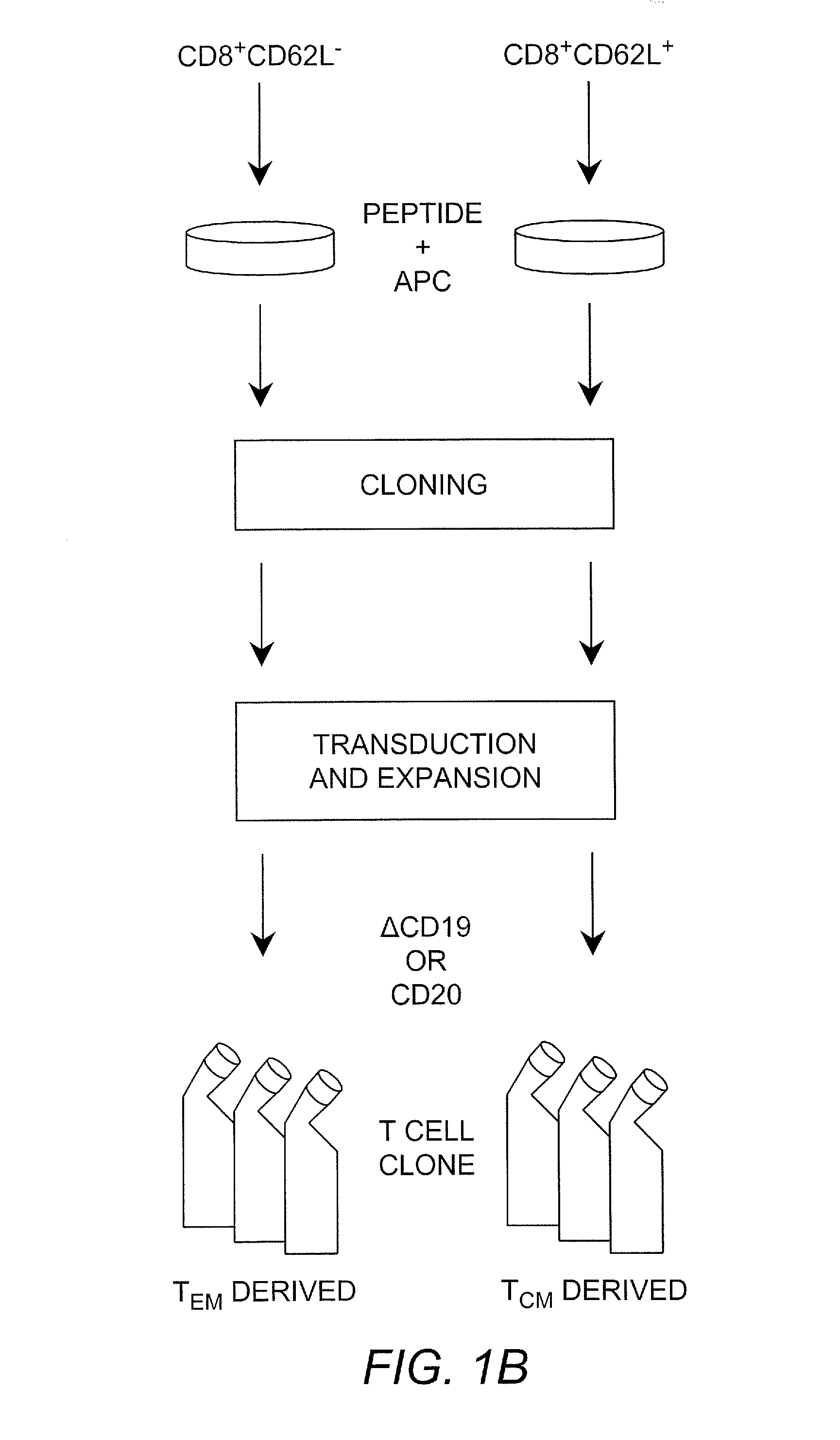
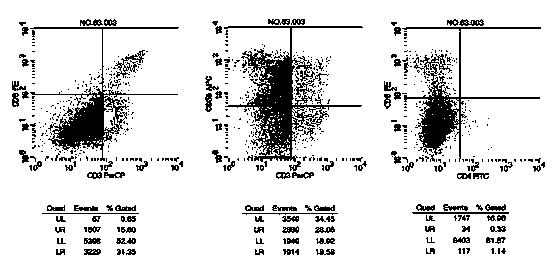
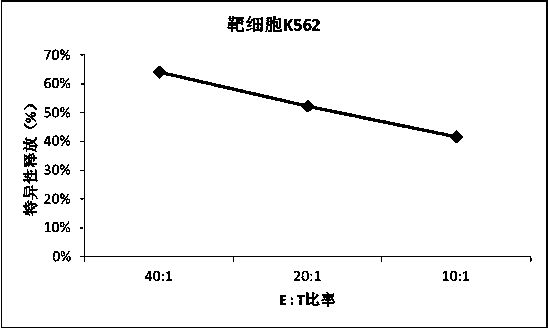
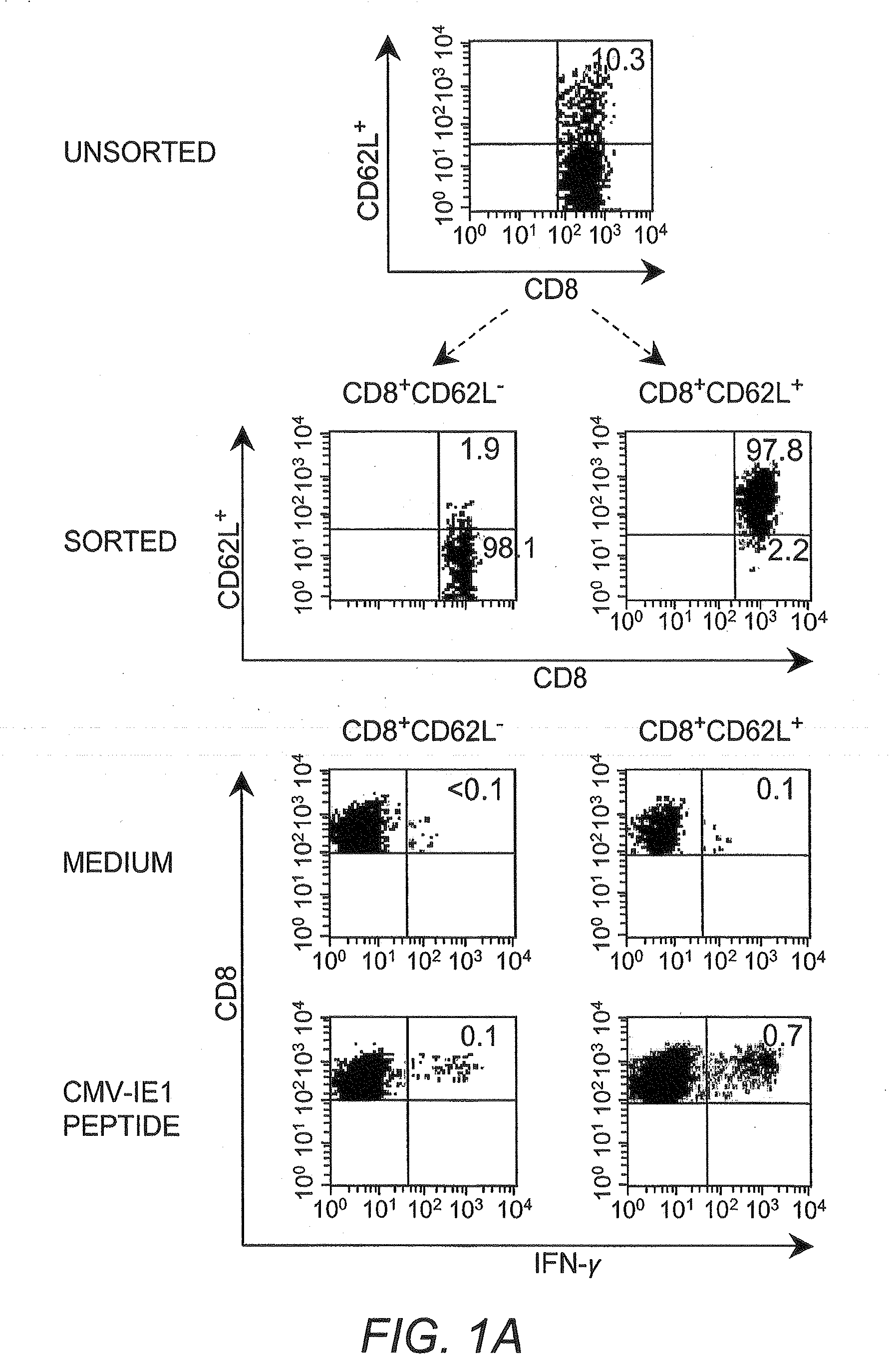
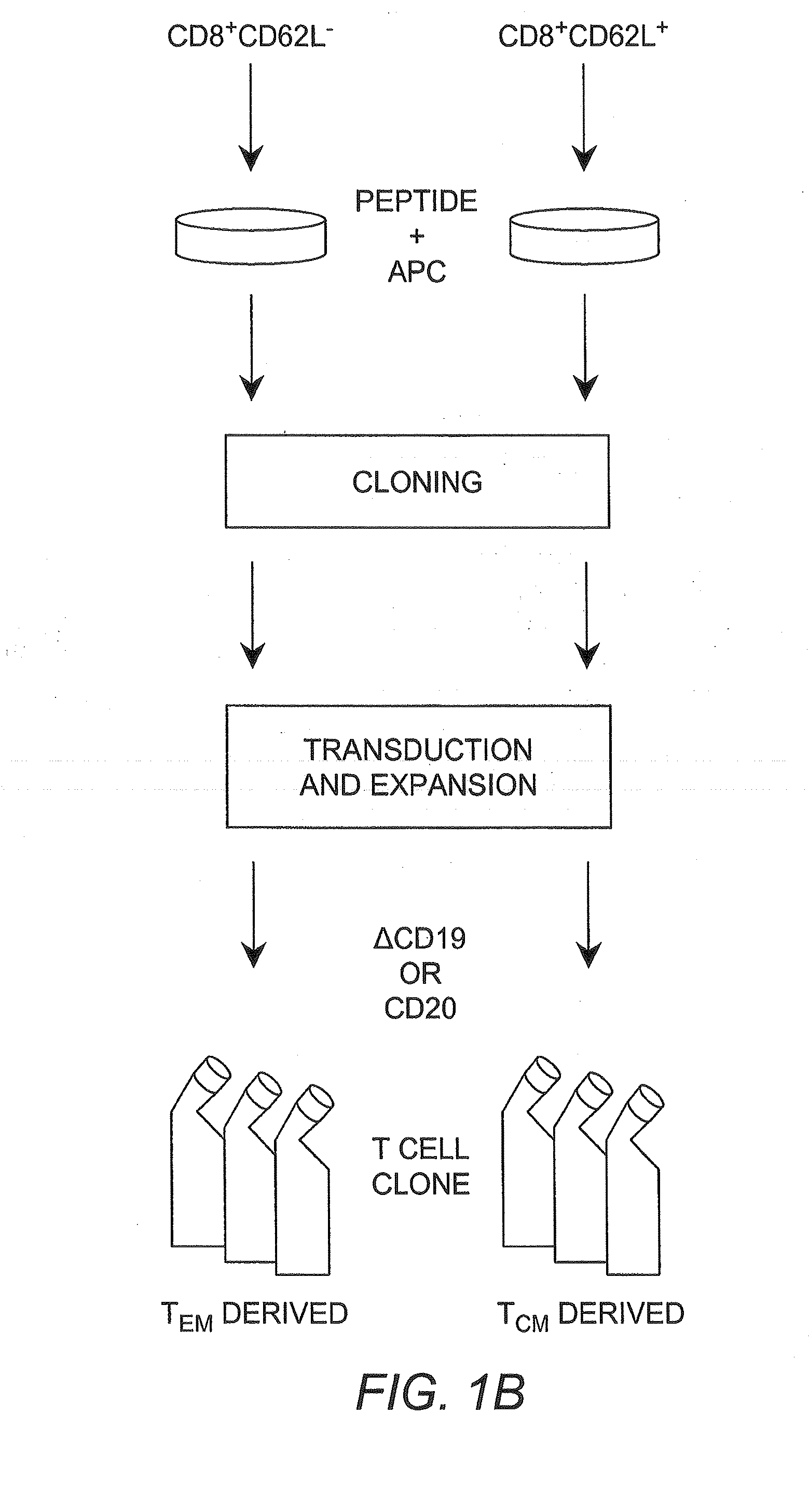
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
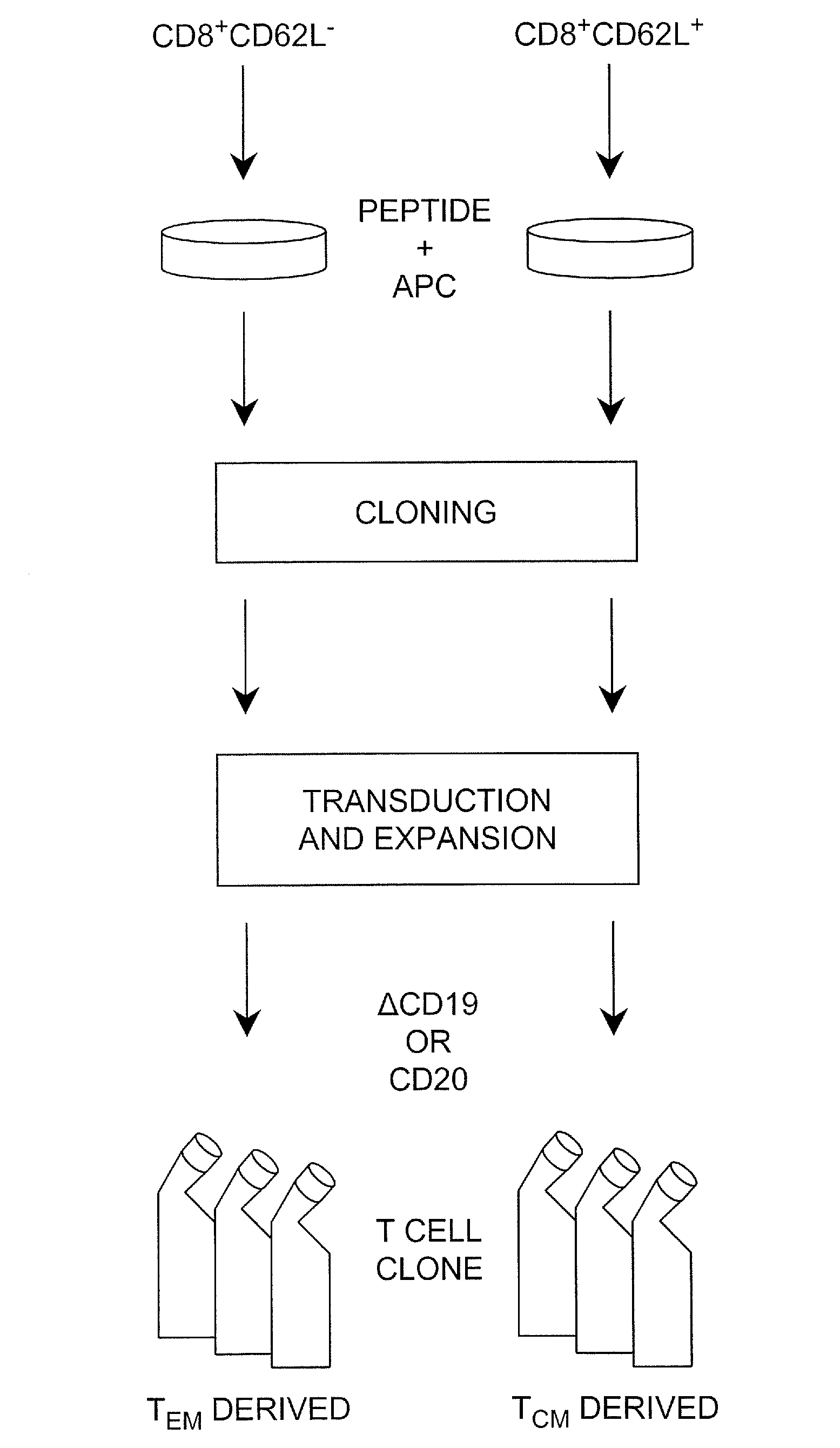
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
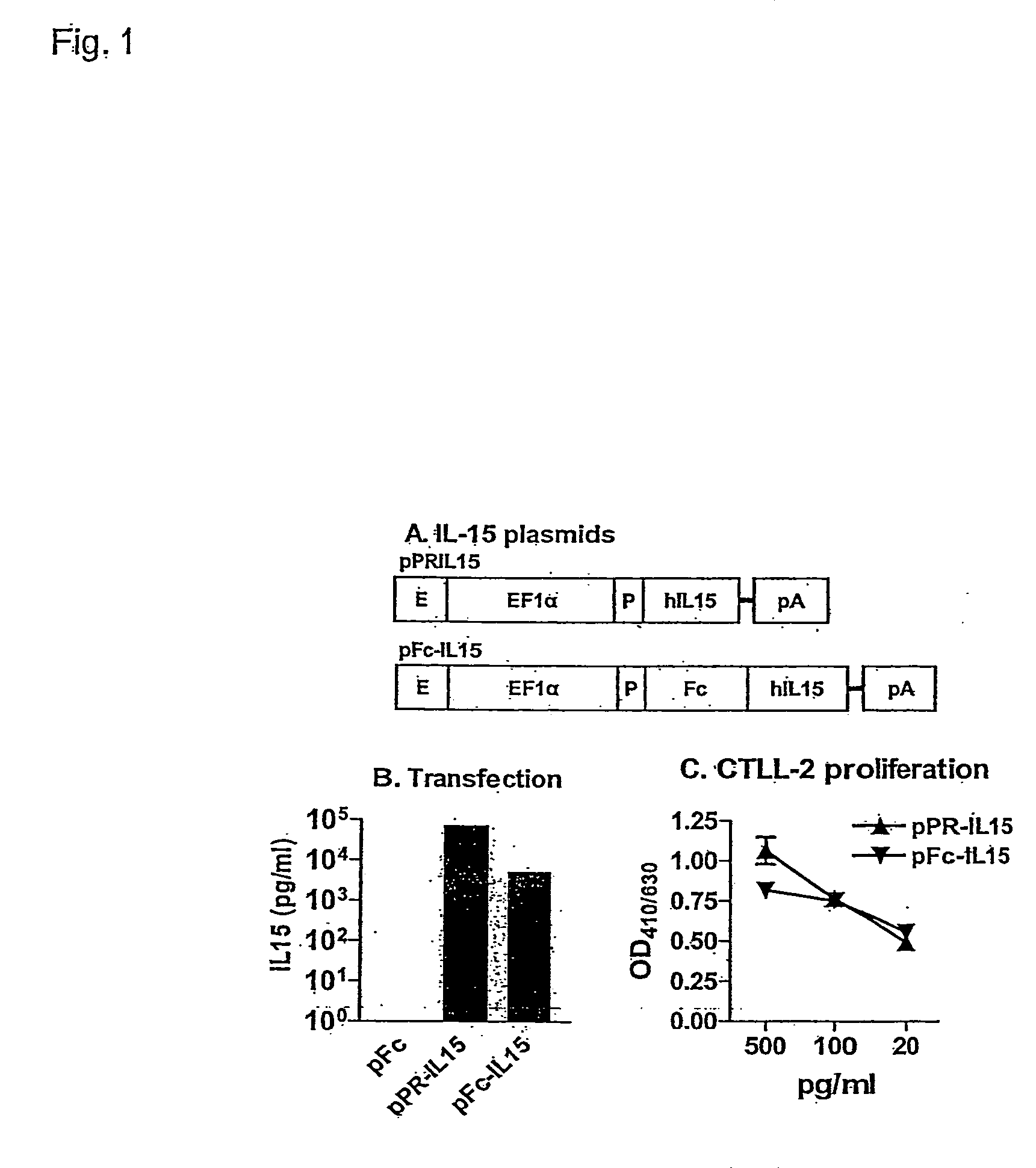
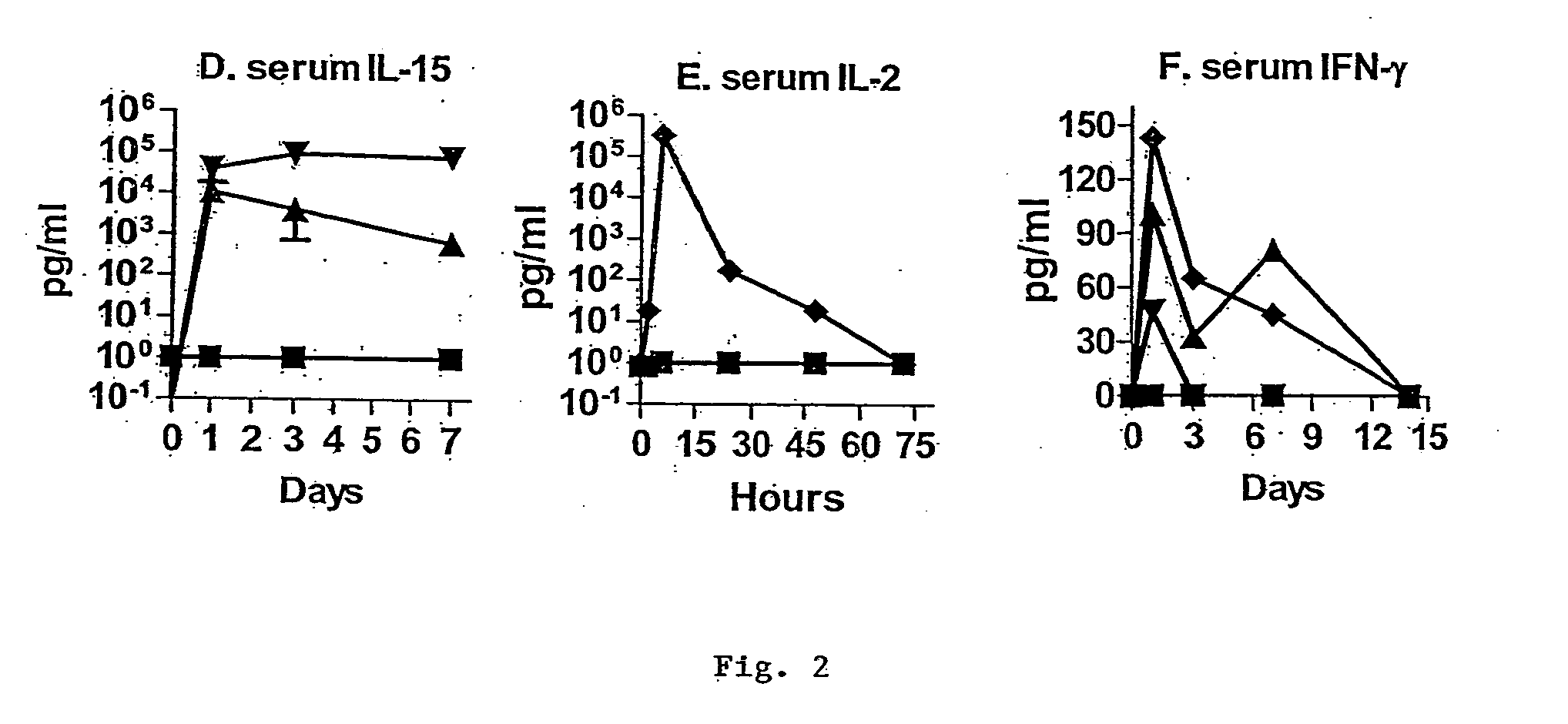
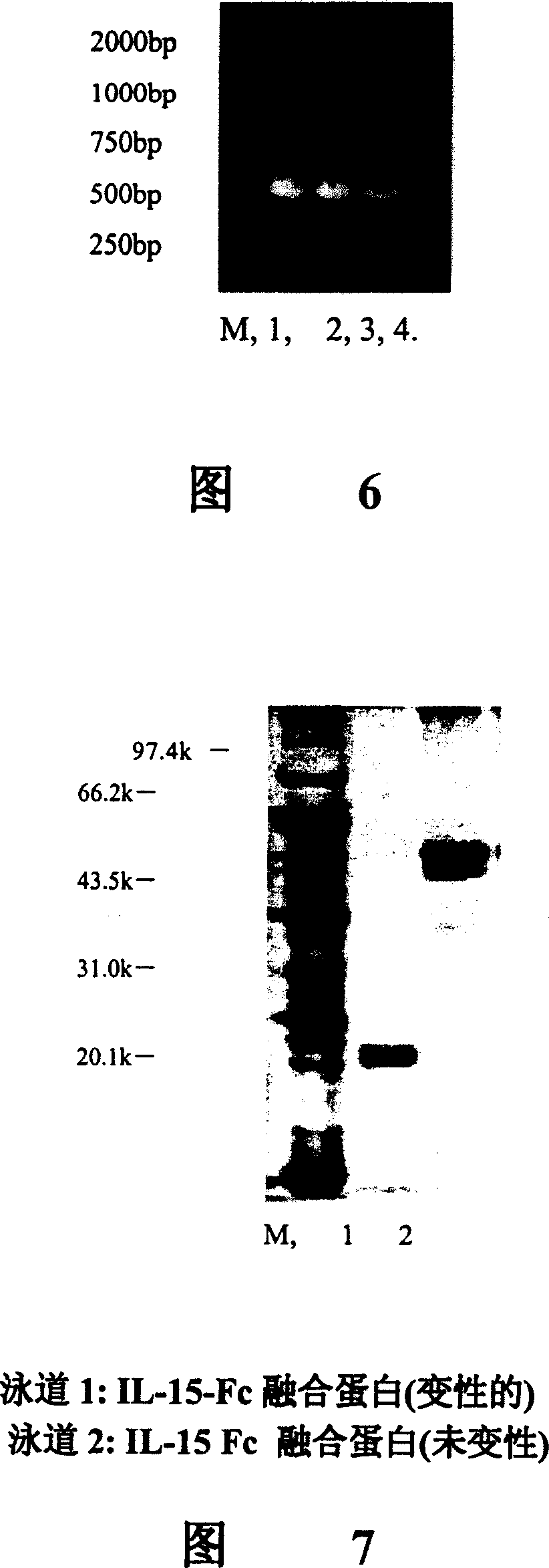
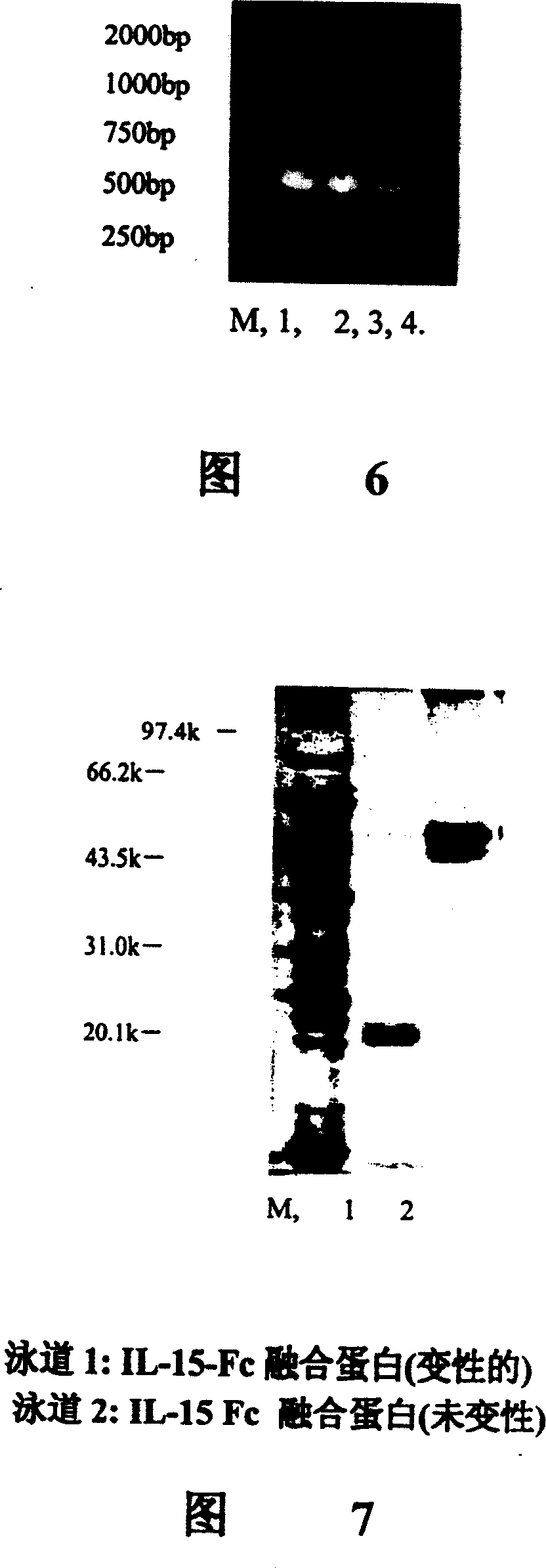
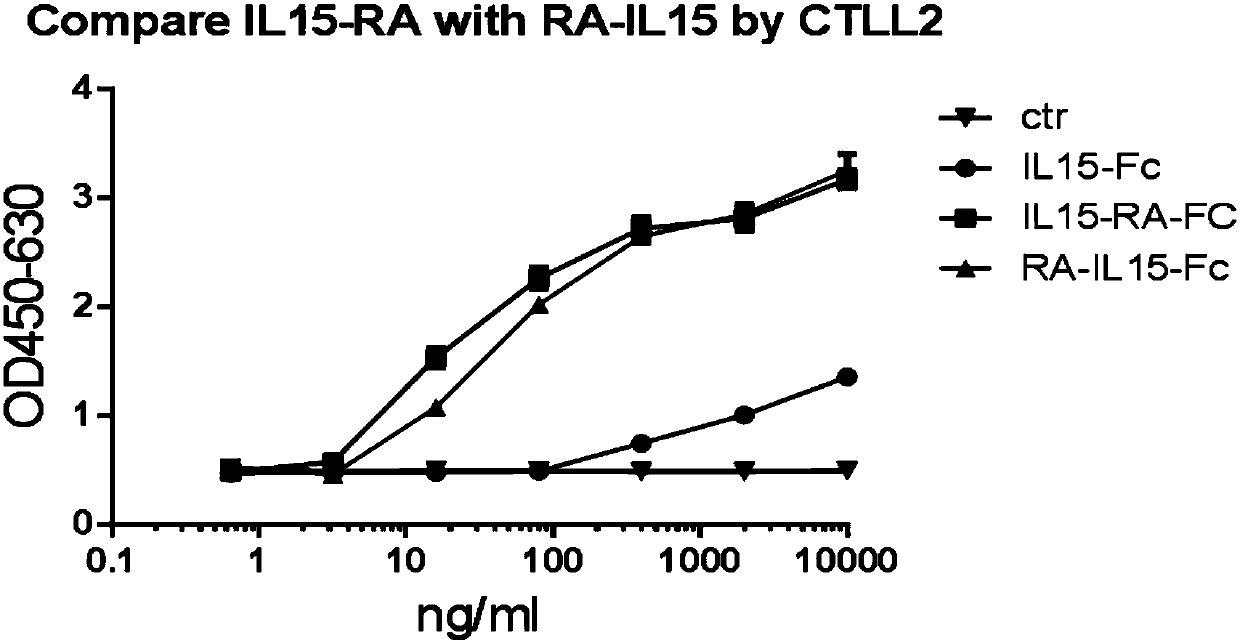
Novel form of interleukin-15, Fc-IL-15, and methods of use
InactiveUS20060257361A1Eliminates any neoantigenicityShort half-lifeHybrid immunoglobulinsPeptide/protein ingredientsAbnormal tissue growthT cell
The present invention relates to Fc-IL-15 hybrids, which may or may not include peptide linkers between the IL-15 and the Fc portion, for methods of treatment of tumors and viral infections. The IL-15 hybrids can be Fc-IL-15 or IL-15-Fc hybrids. The Fc-IL-15 hybrids include variants, including the IL-15 and Fc variants. The hybrids preferably (but not necessarily) include peptide linkers between the IL-15 and the Fc portion. These linkers are preferably composed of a T cell inert sequence, or any non-immunogenic sequence.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
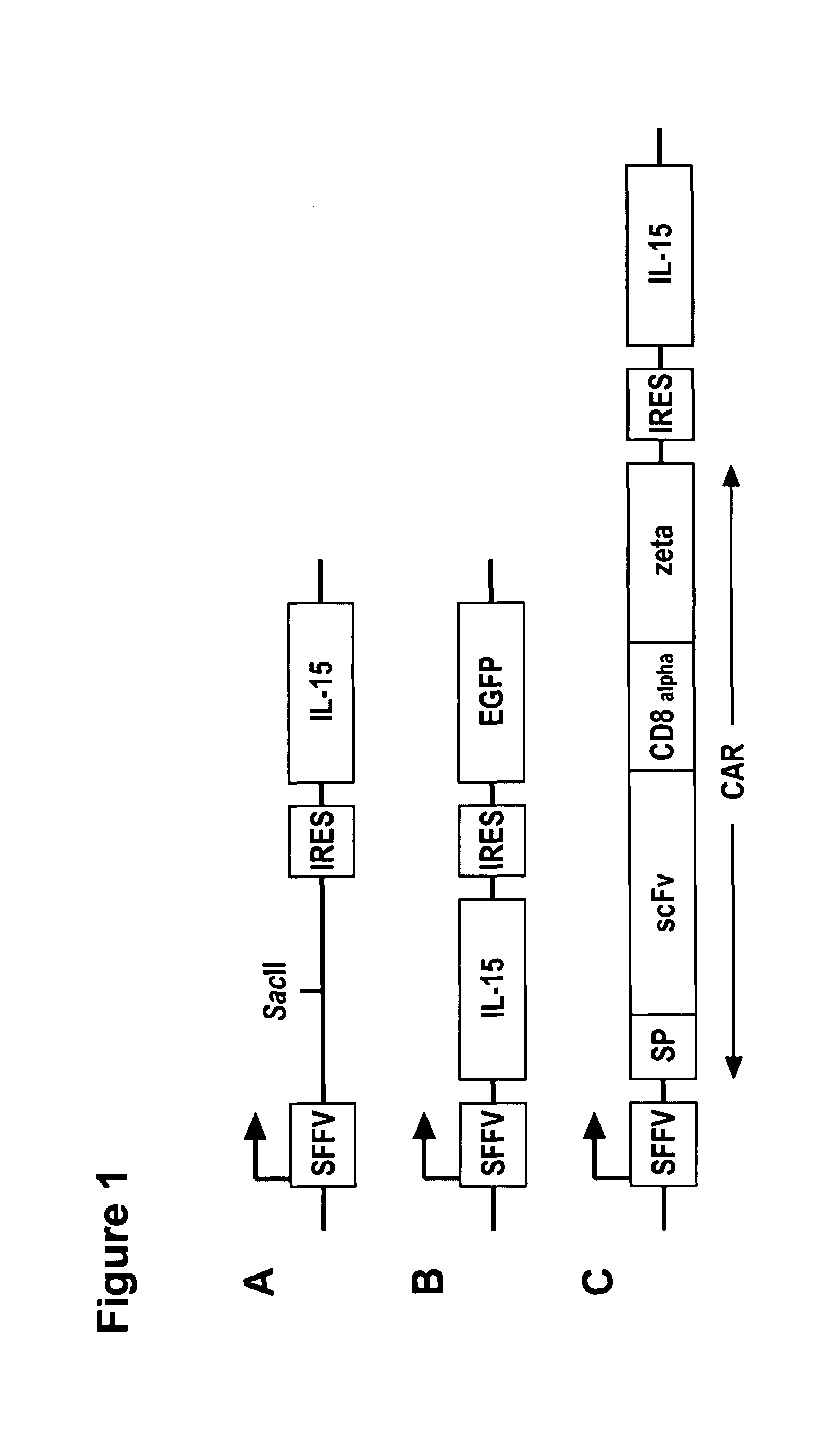
Interleukin 15 as Selectable Marker for Gene Transfer in Lymphocytes
ActiveUS20130280221A1Opening possibilityBiocideAntibody mimetics/scaffoldsADAMTS ProteinsAntigen receptors
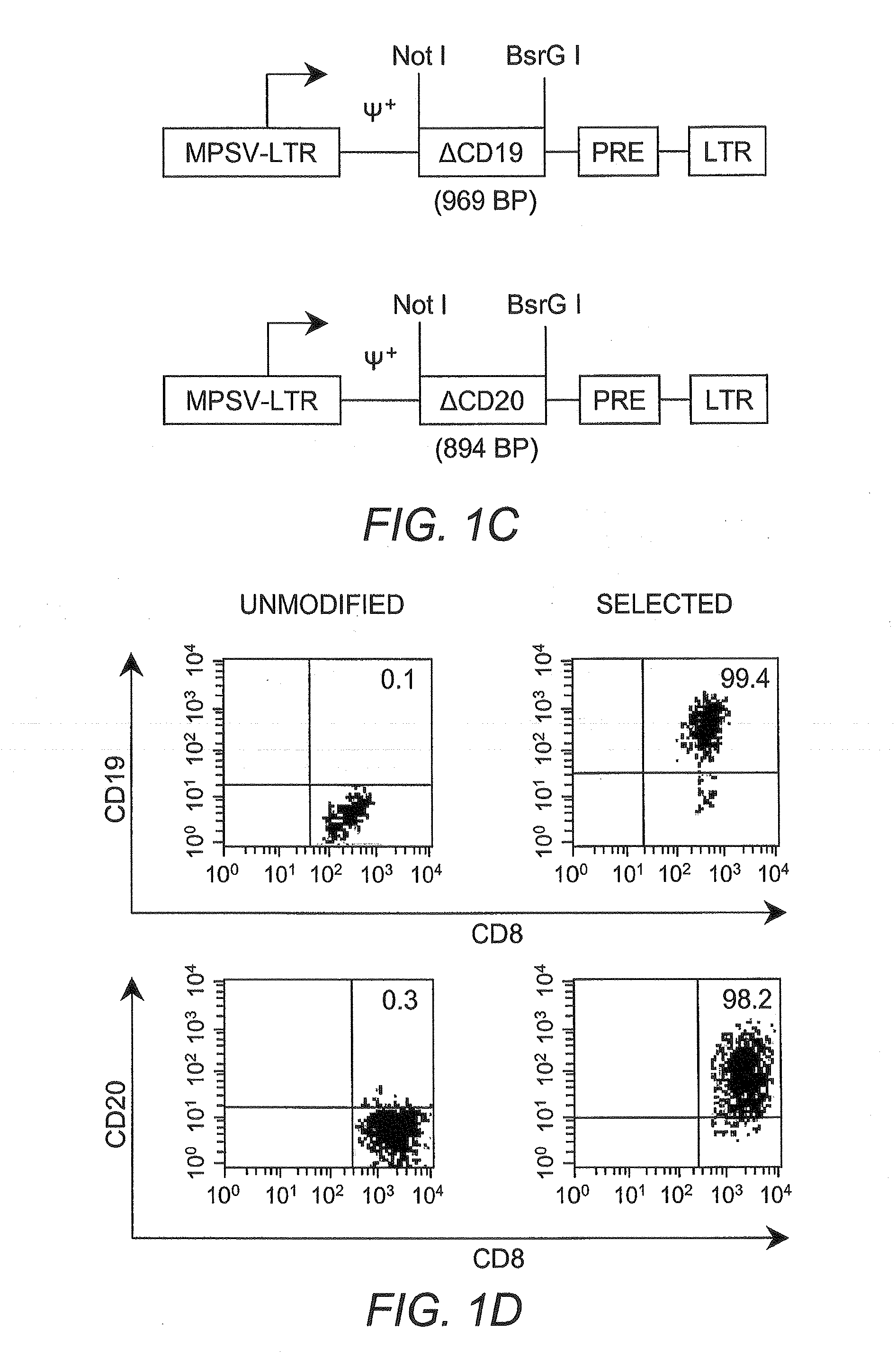
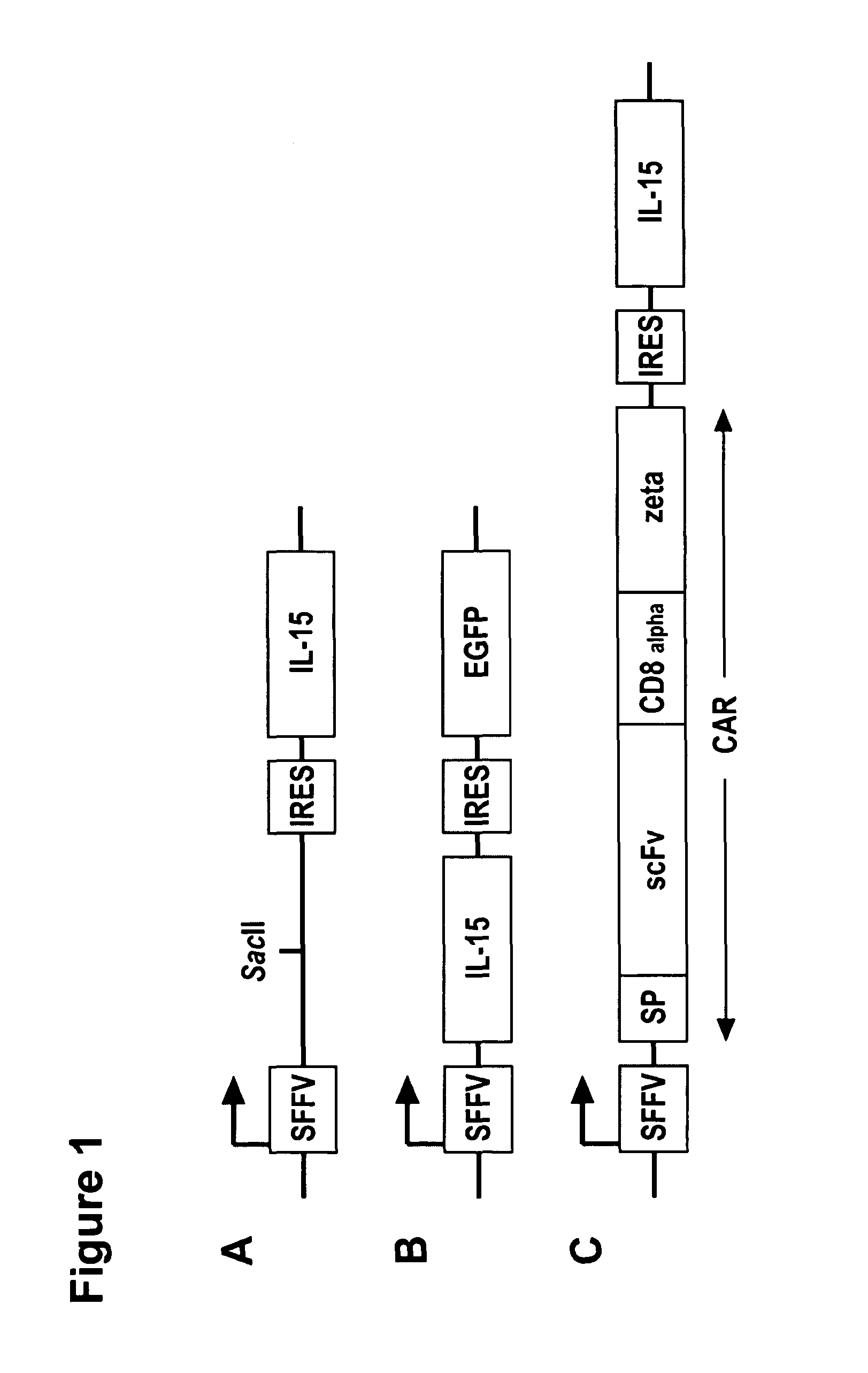
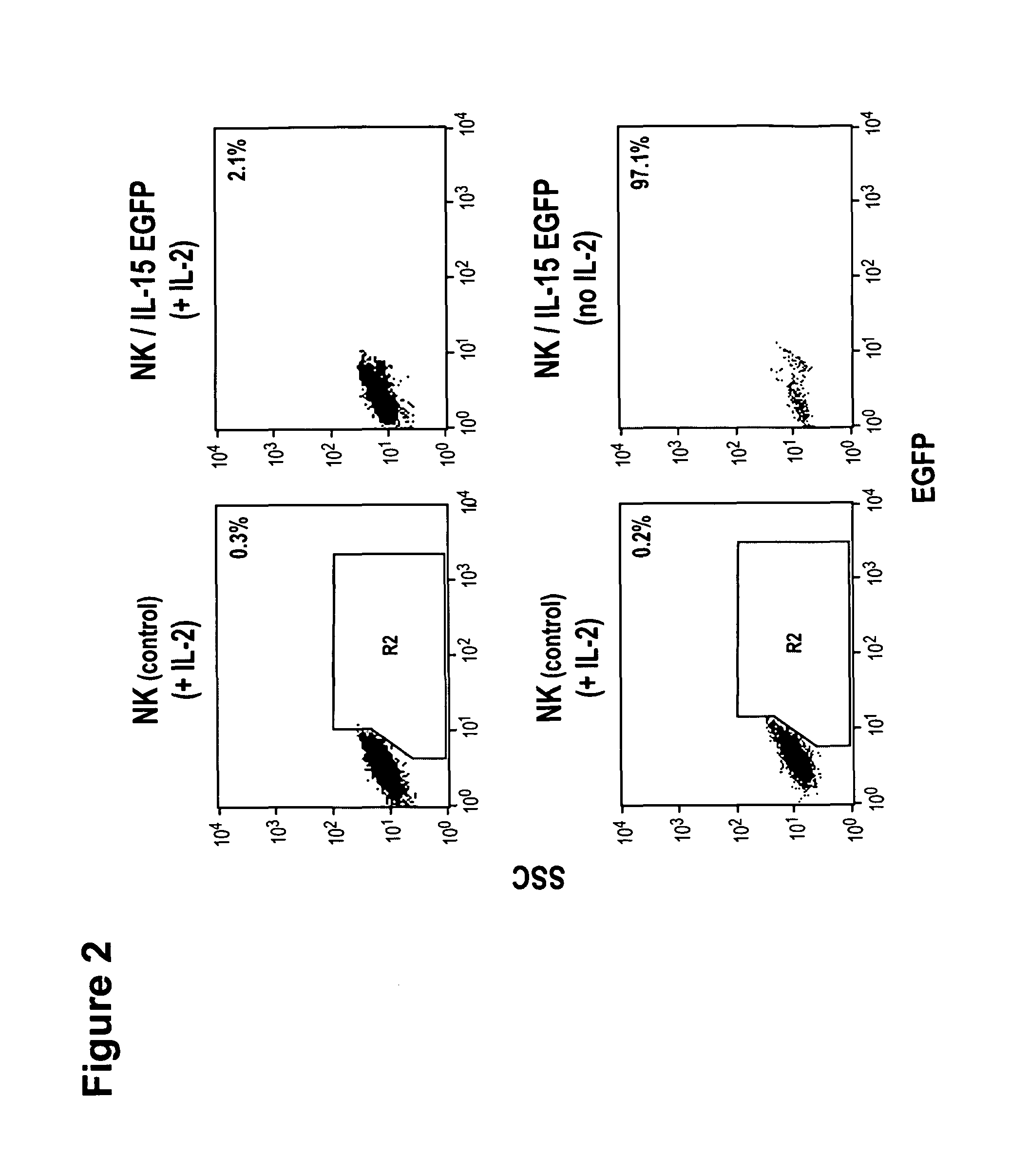
The present invention relates to the use of interleukin-15 (IL-15) as selectable marker for gene transfer, preferably of at least one gene of therapeutic interest, into a mammalian cell or cell line, in particular a human cell or cell line. The present invention furthermore relates to transgenic mammalian cells or cell lines expressing IL-15 as selectable marker and co-expressing at least one protein of interest encoded by at least one gene of interest, which is preferably a protein of therapeutic interest. The present invention is in particular suitable for chimeric antigen receptors (CARs) as the gene or protein of interest and their expression in lymphocytes. The transgenic mammalian cells and cell lines are furthermore suitable for use as a medicament, in particular in the treatment of cancer and in immunotherapy, such as adoptive, target-cell specific immunotherapy.
Owner:CHEMOTHERAPEUTISCHES FORSCHUNGINSTITUT GEORG SPEYER HAUS
Interfusion protein of human interleukin 15 and Fe
ActiveCN1760209APeptide/protein ingredientsAntibody ingredientsWhite blood cellImmunoglobulin Fc Fragments
A human interleukin 15-Fc fusion protein composed of interleukin 15 and the Fc fragment of human immunoglobulin, which are linked via joining peptide, the nucleic acid ofr coding it, the expression carrier containing said nucleic acid, its composite medicine for preventing and treating microbial infection and its preparing process are disclosed.
Owner:上海海欣生物技术有限公司
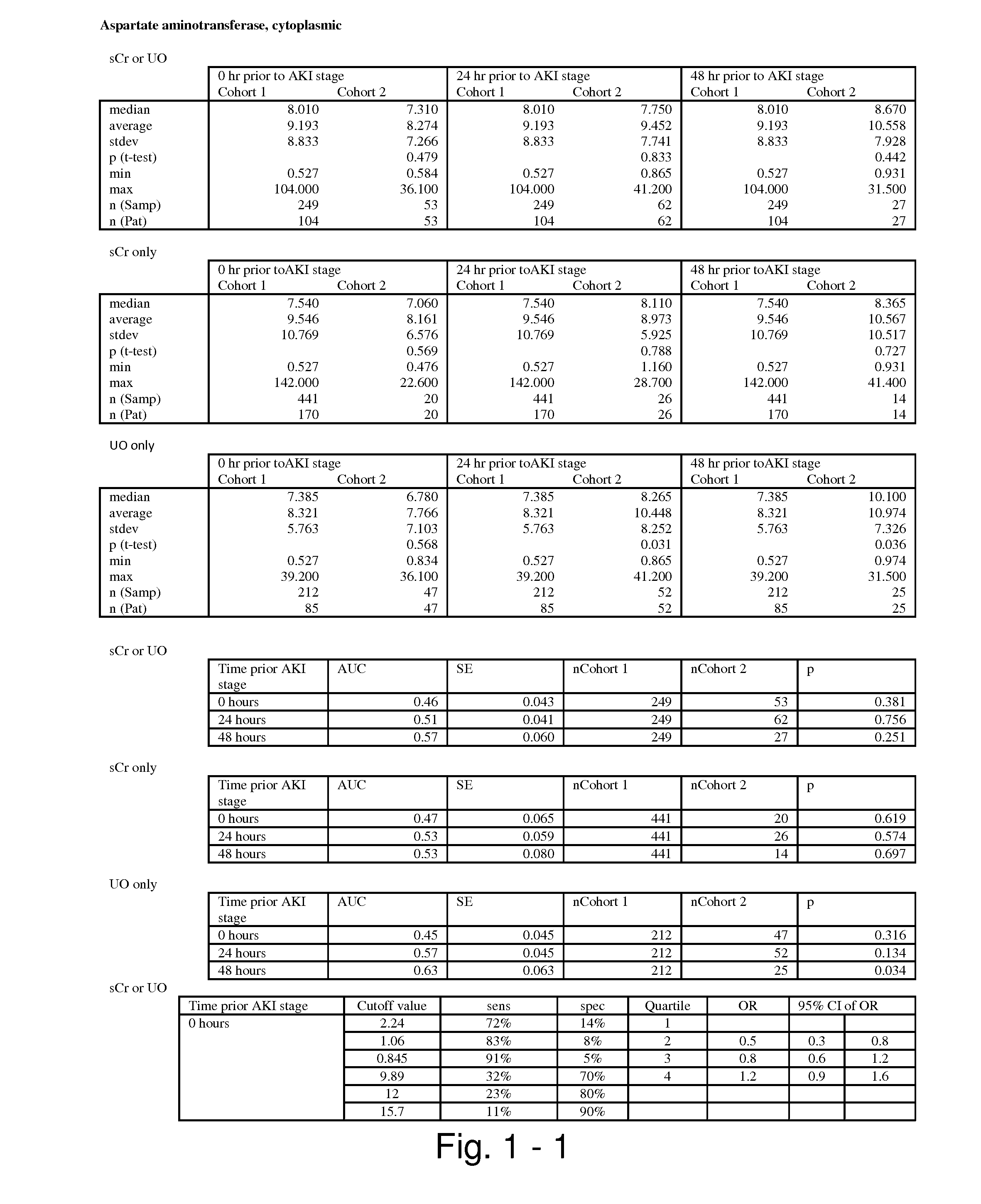
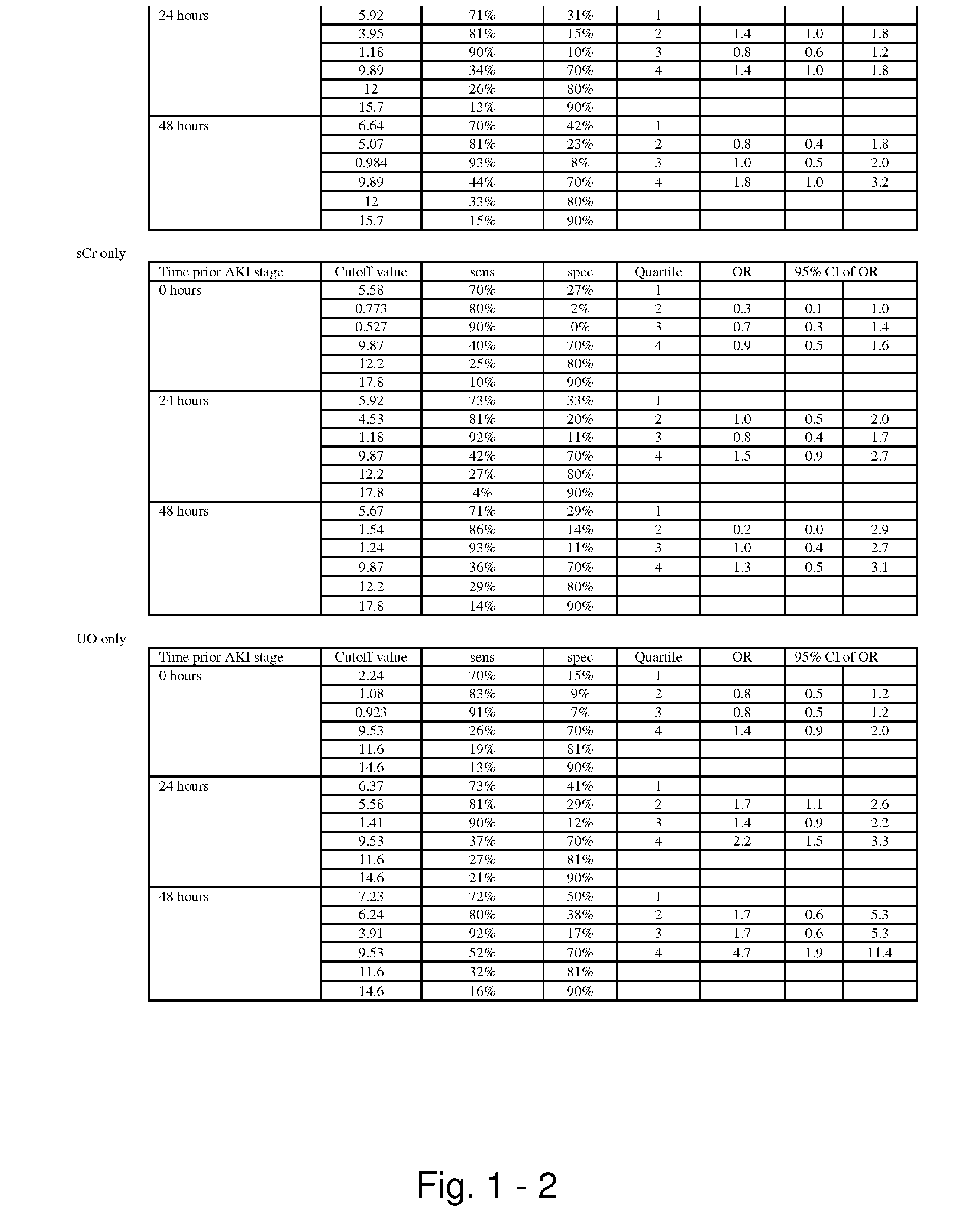
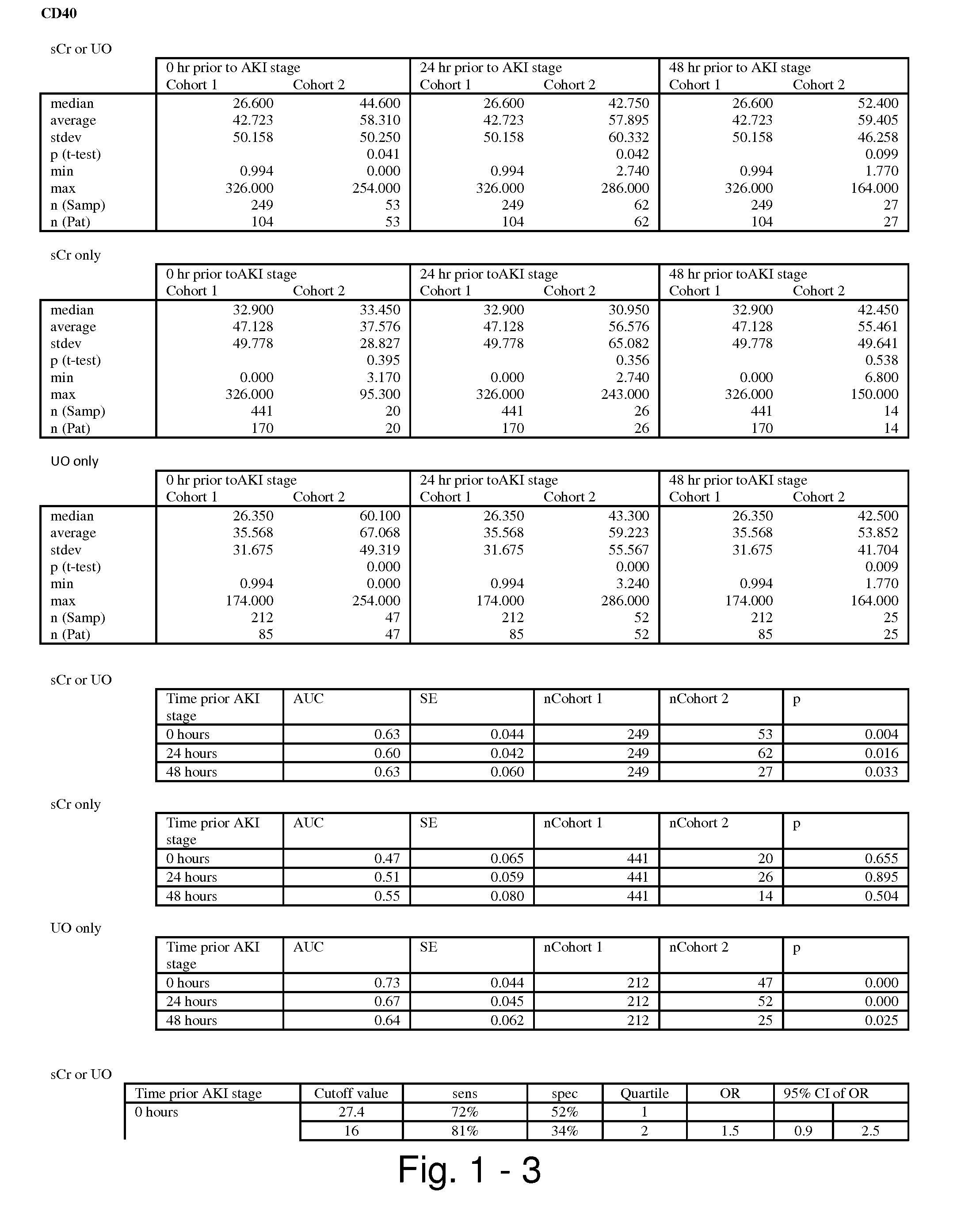
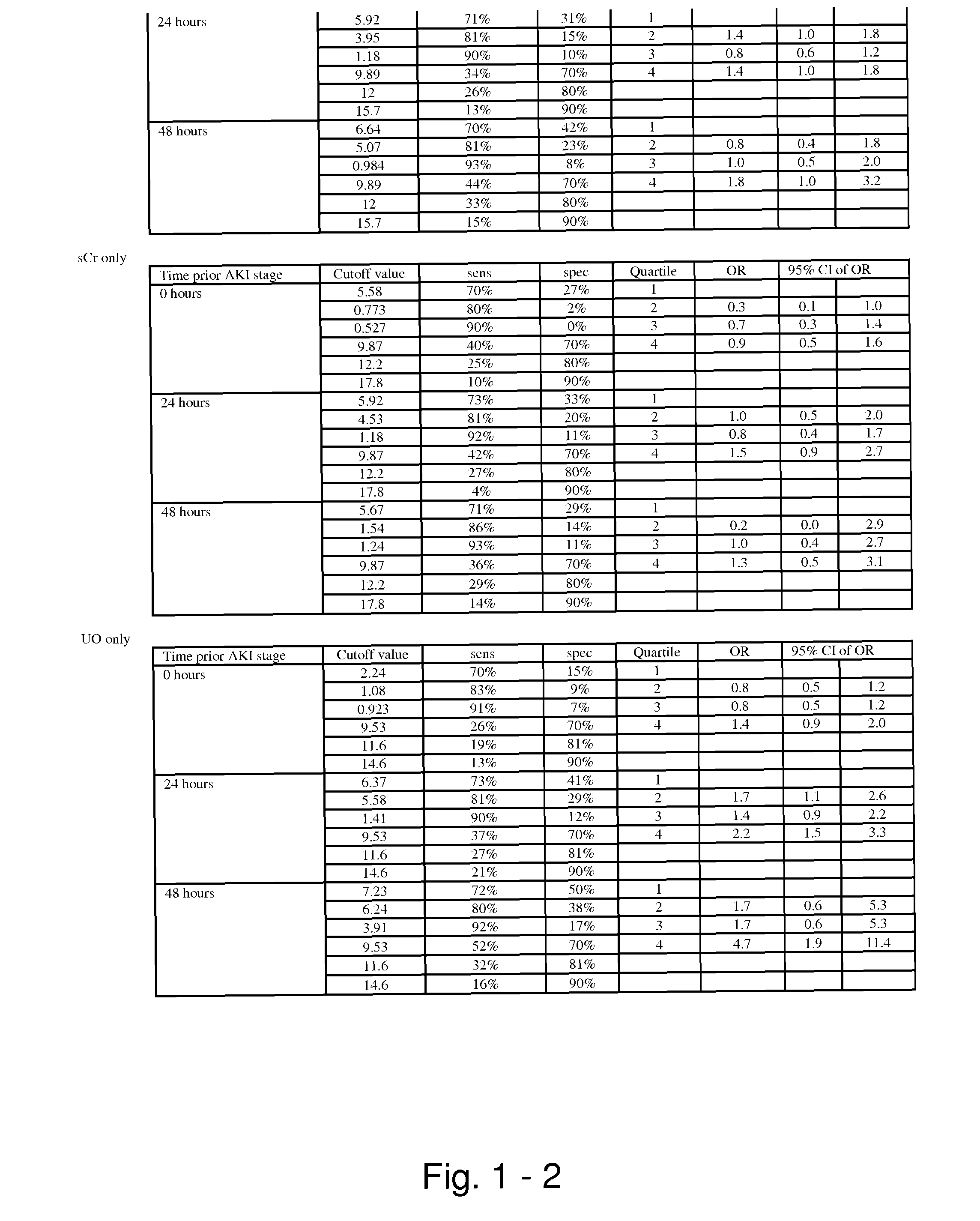
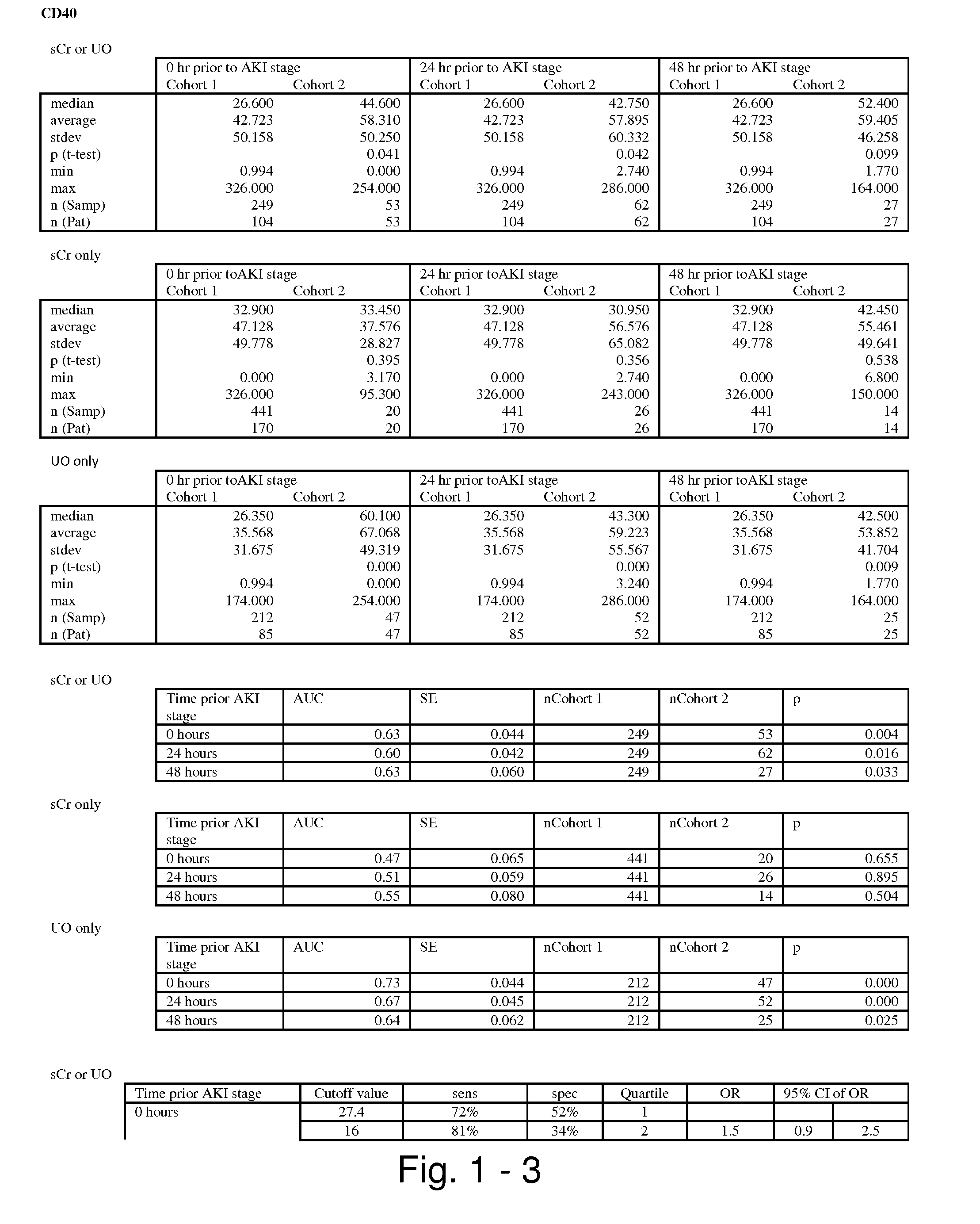
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
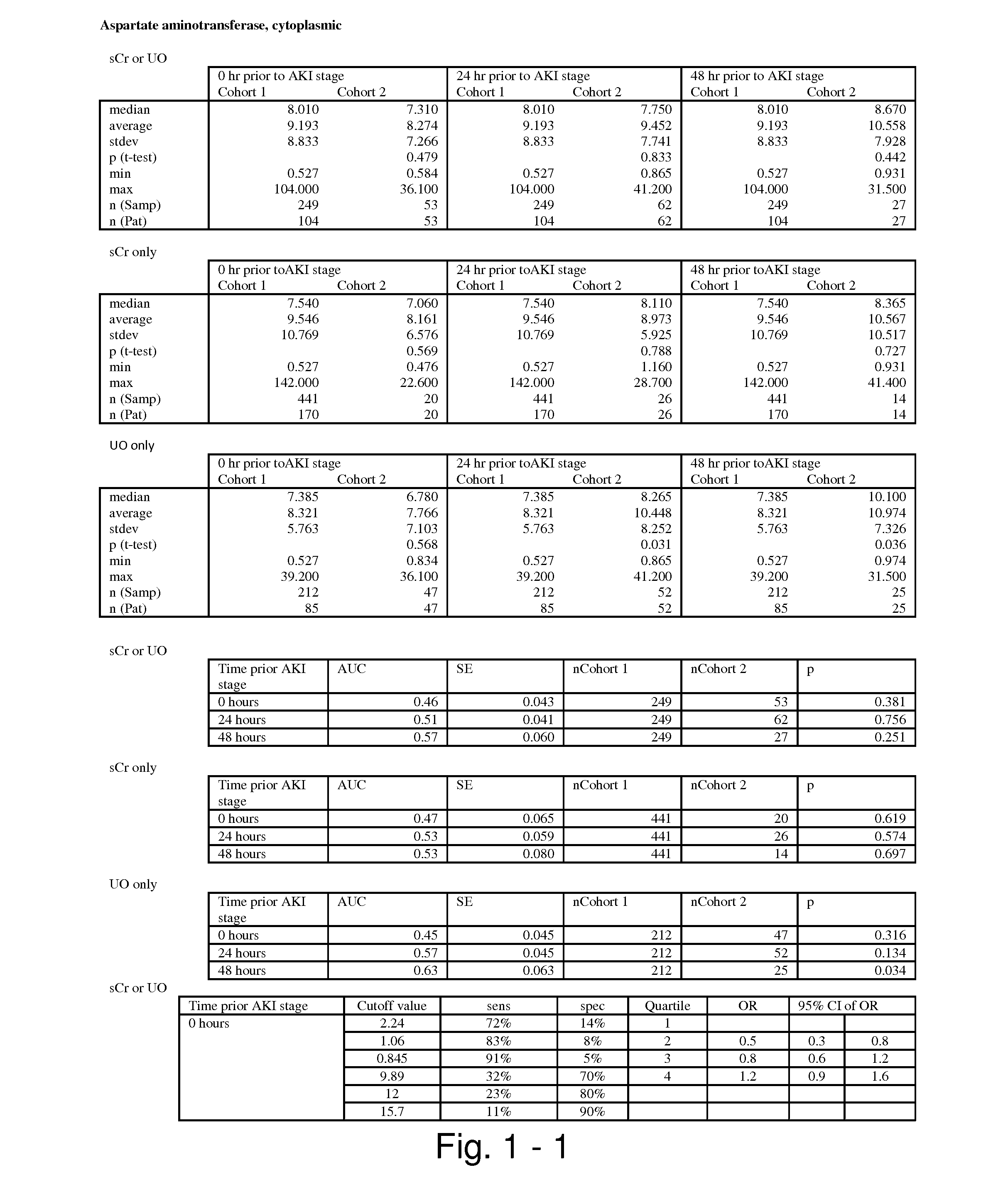
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
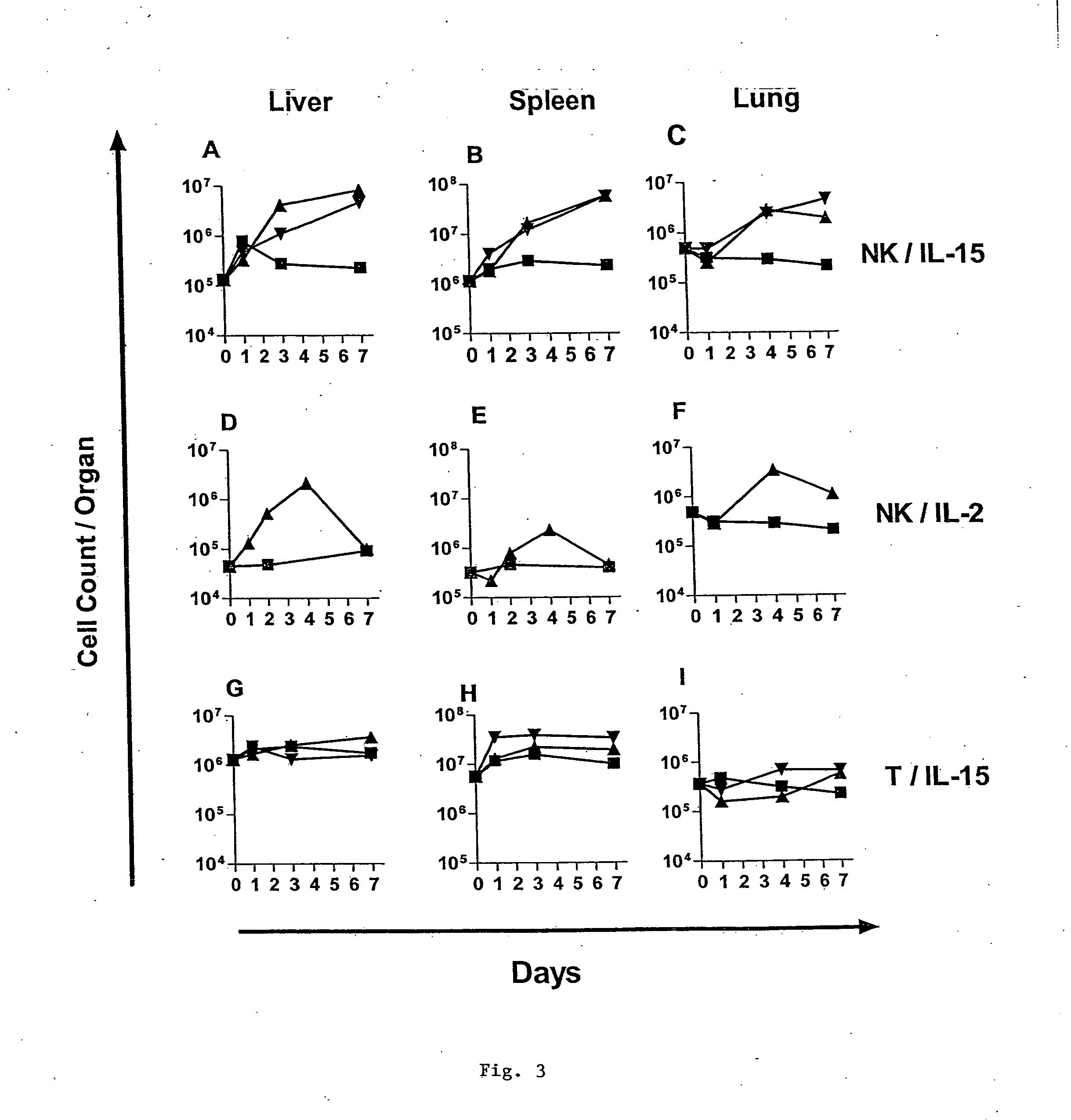
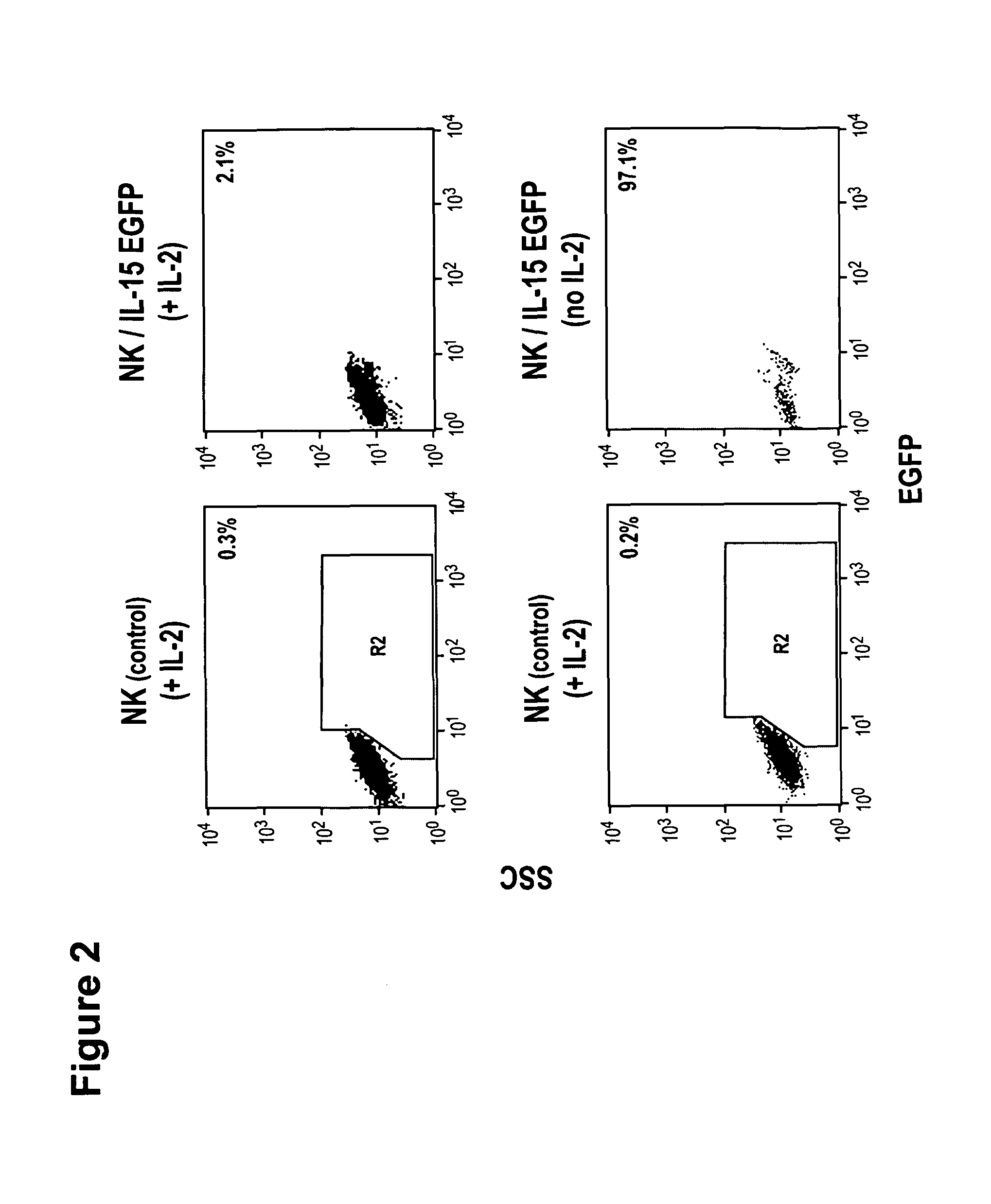
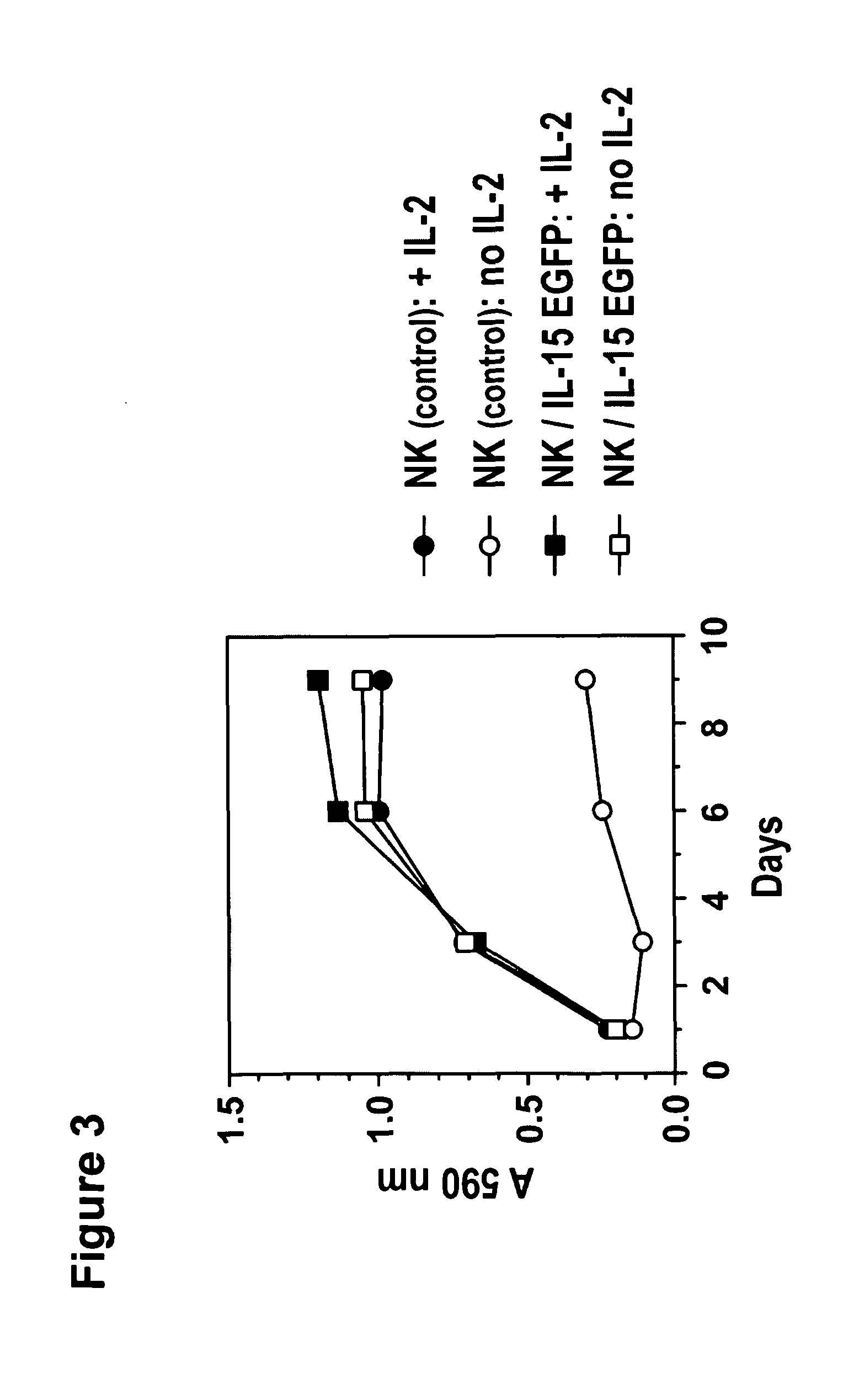
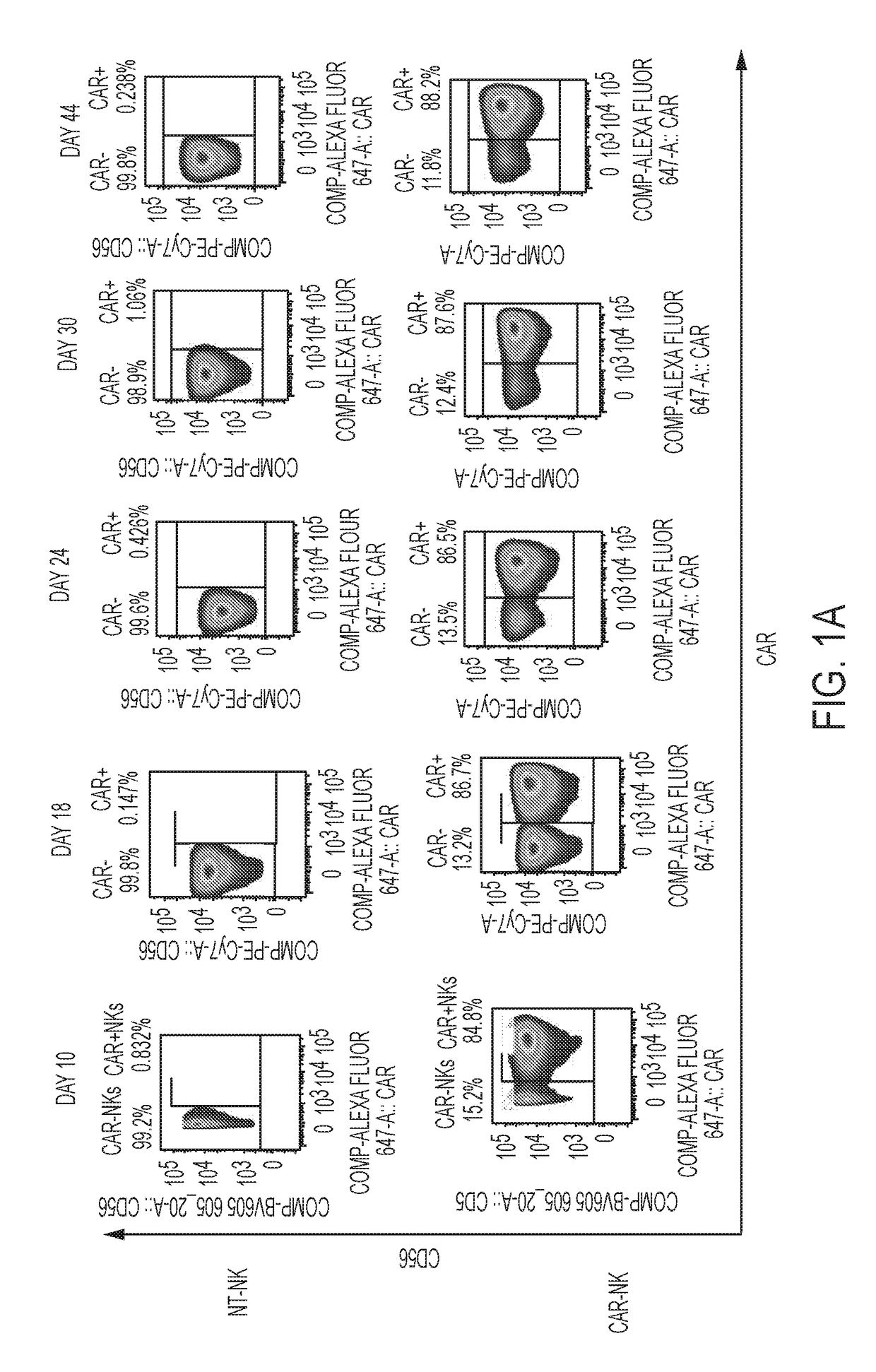
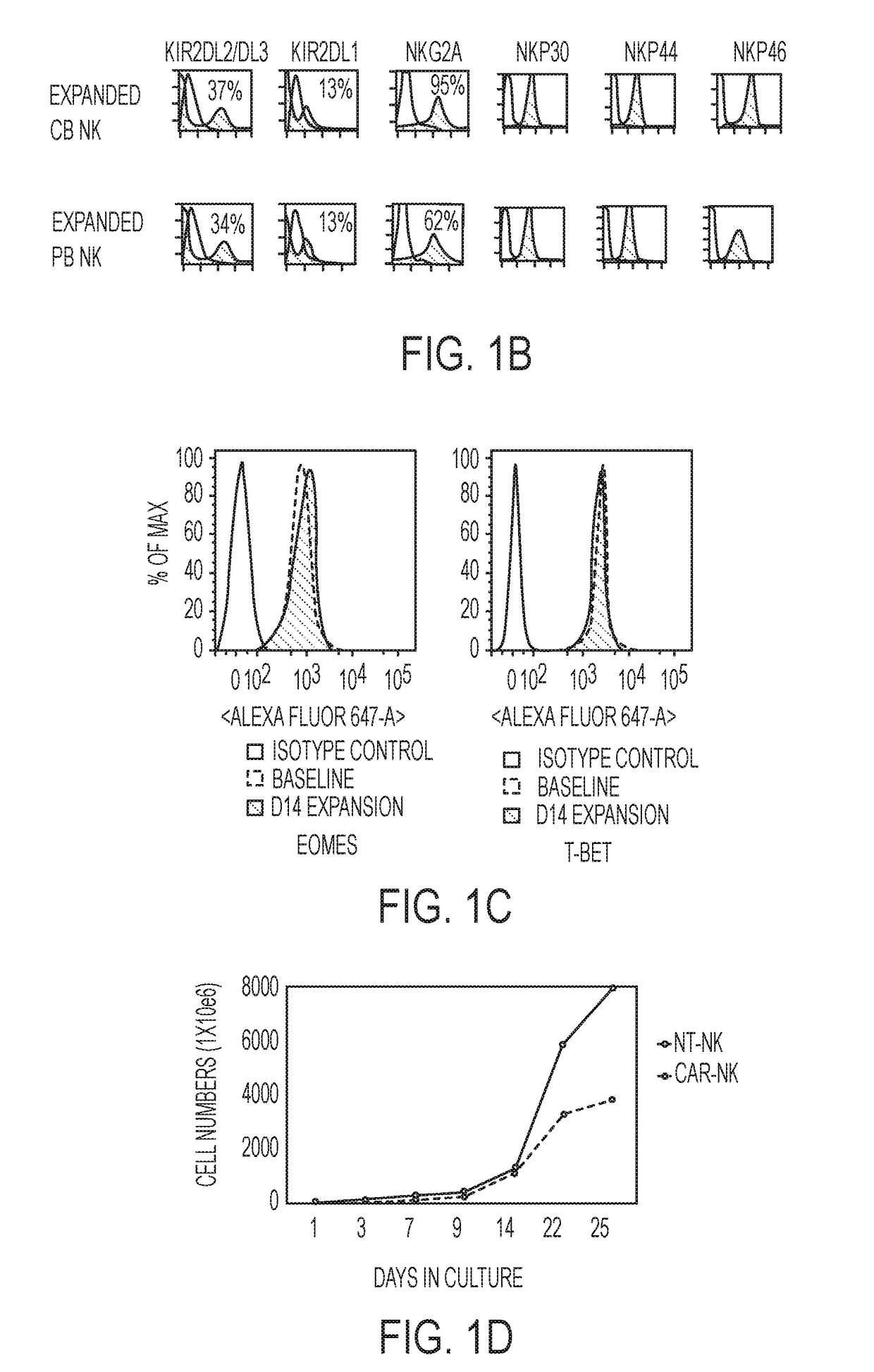
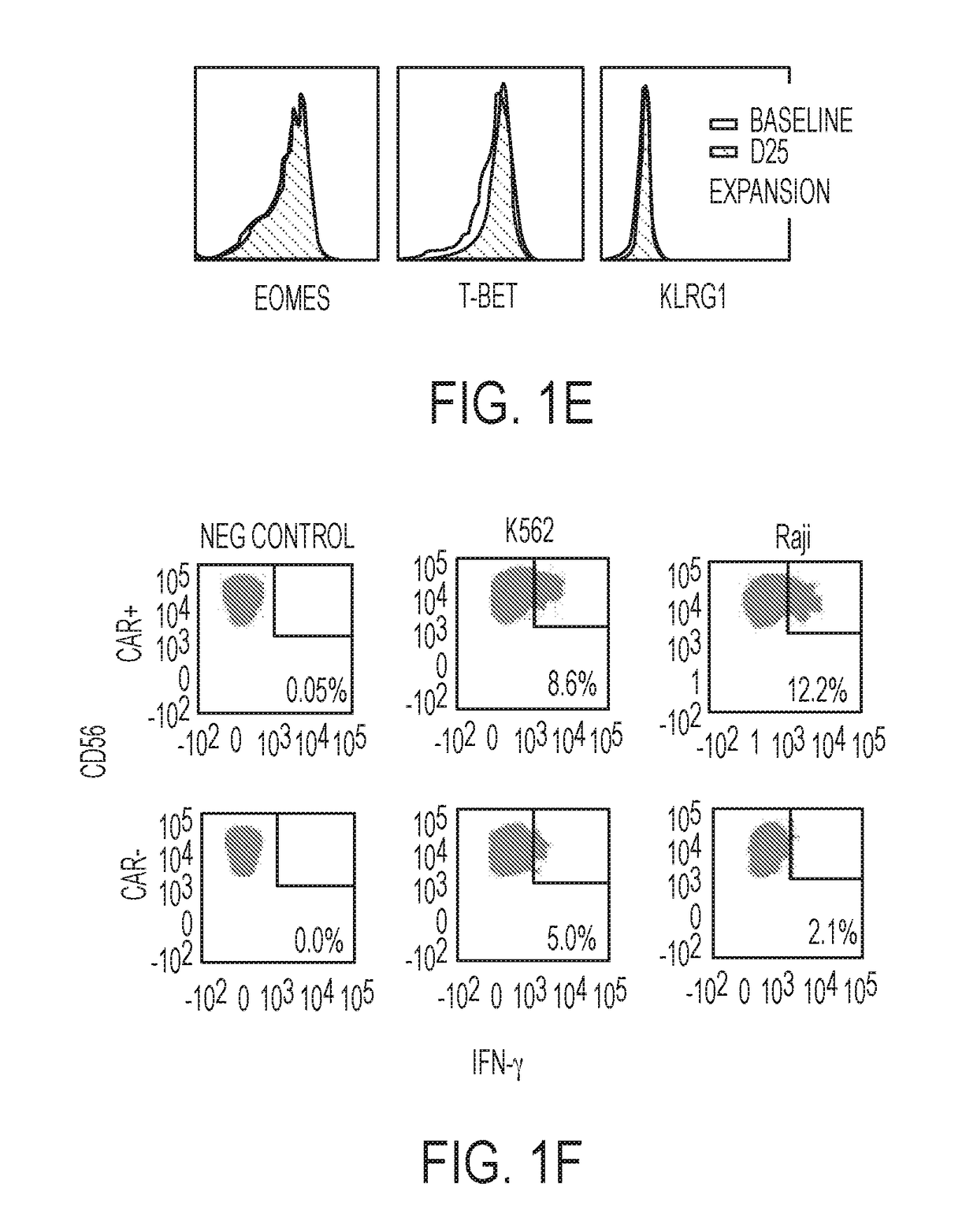
Modified Natural Killer Cells and Uses Thereof
ActiveUS20170073638A1Polypeptide with localisation/targeting motifCell receptors/surface-antigens/surface-determinantsNatural Killer Cell Inhibitory ReceptorsNatural killer cell
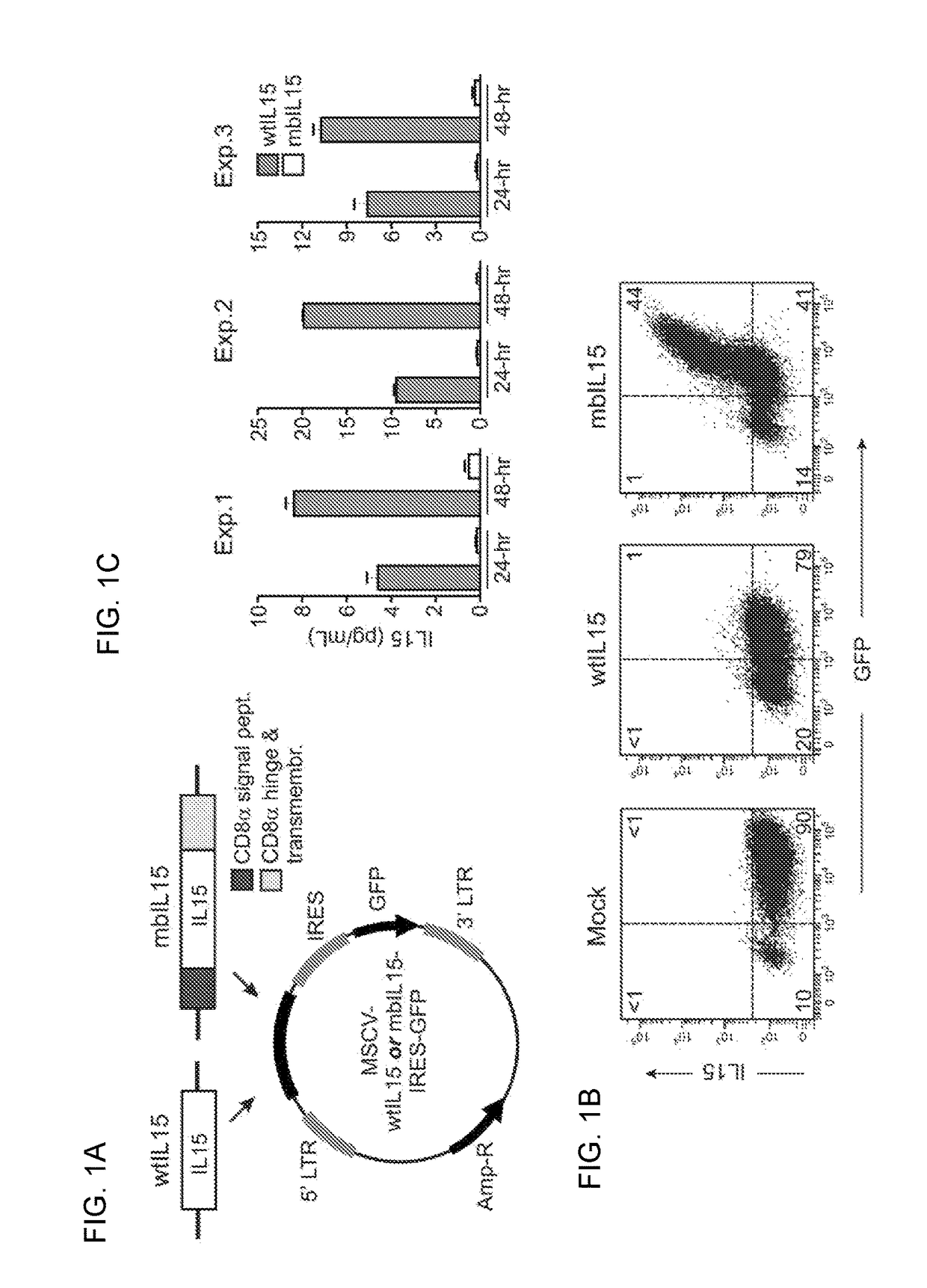
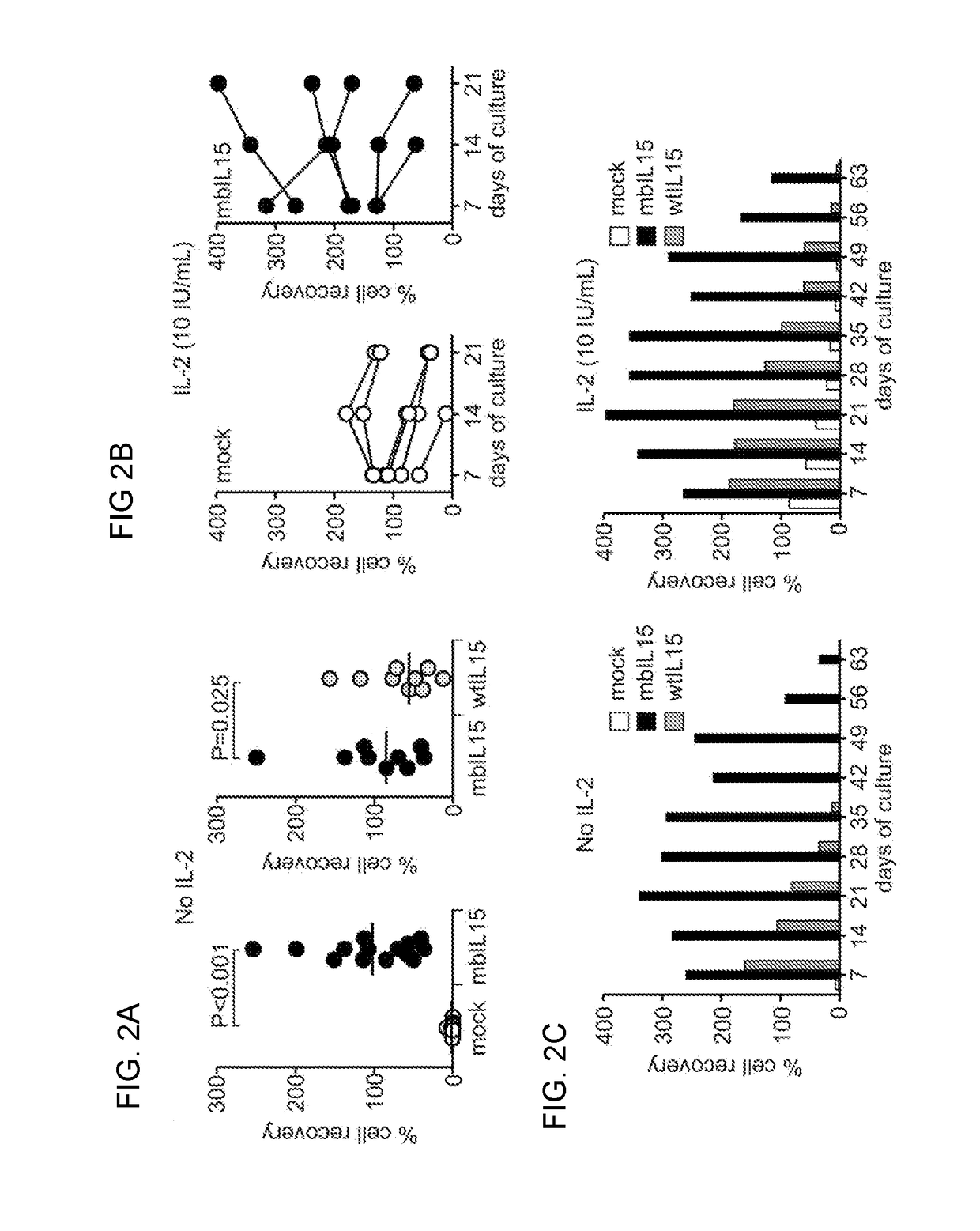
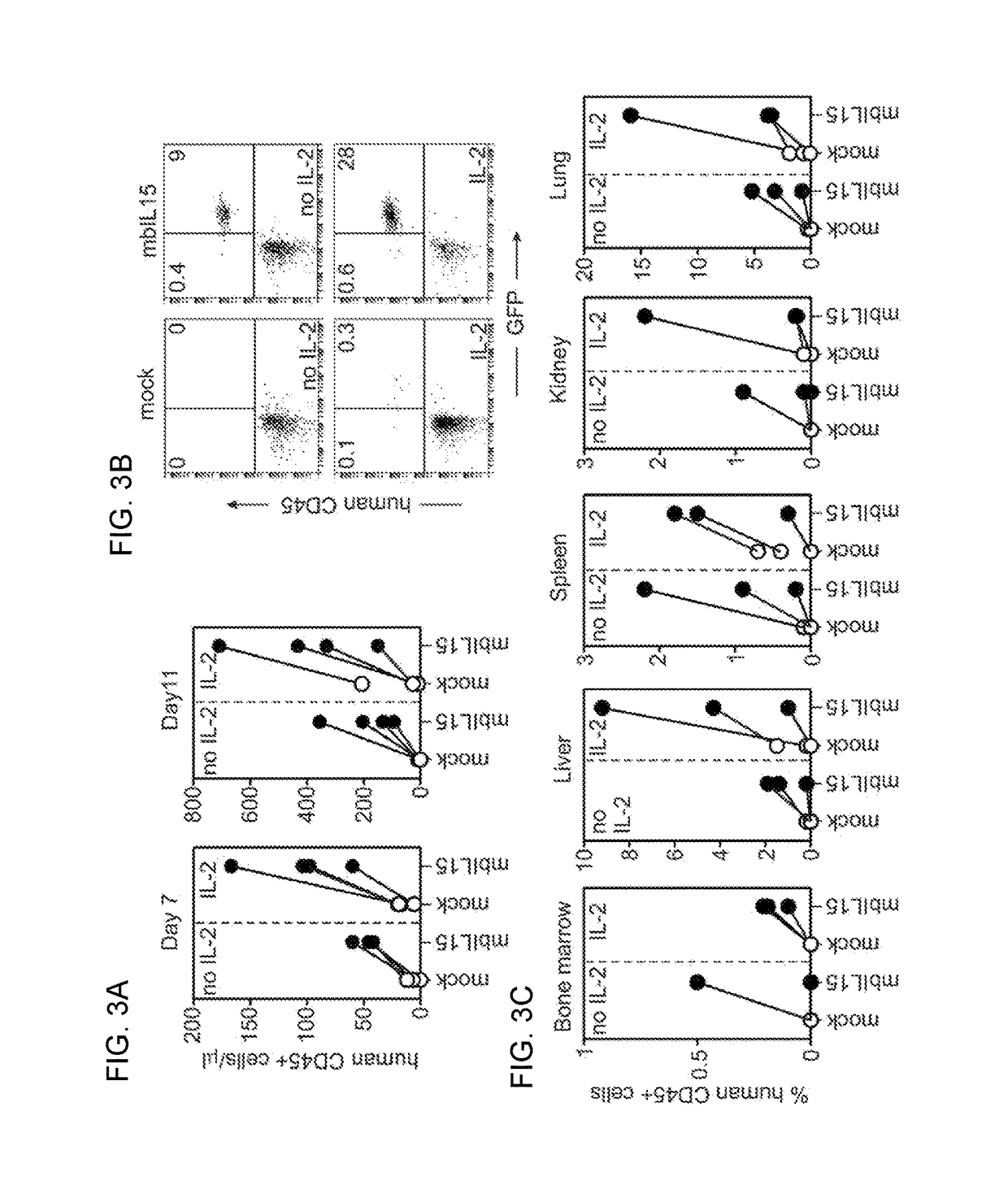
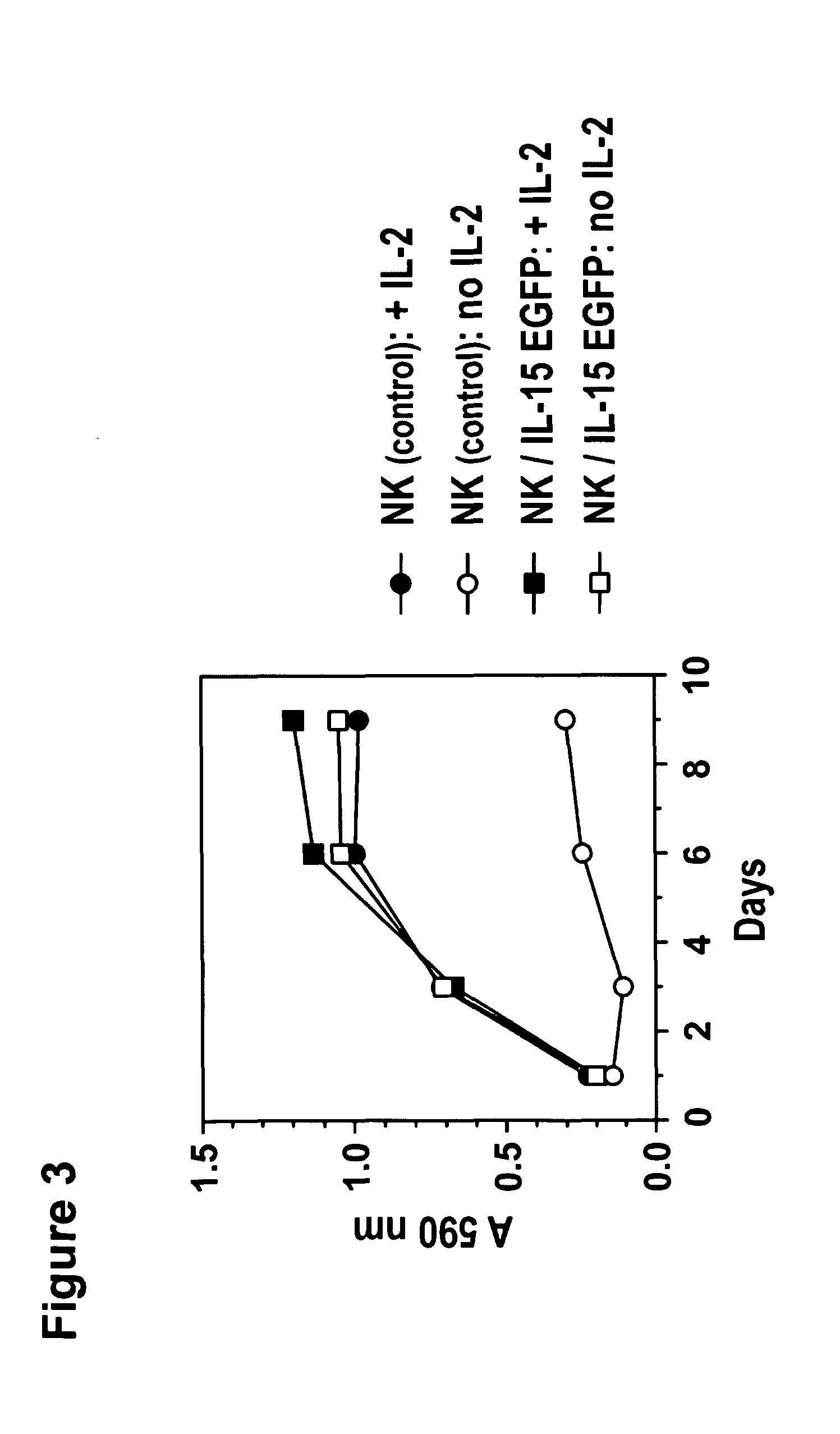
The present invention provides, in certain aspects, a natural killer (NK) cell that expresses all or a functional portion of interleukin-15 (IL-15), and methods for producing such cells. The invention further provides methods of using a natural killer (NK) cell that expresses all or a functional portion of interleukin-15 (IL-15) to treat cancer in a subject or to enhance expansion and / or survival of NK cells.
Owner:NAT UNIV OF SINGAPORE +1
Nucleic acids encoding antagonists of interleukin-15
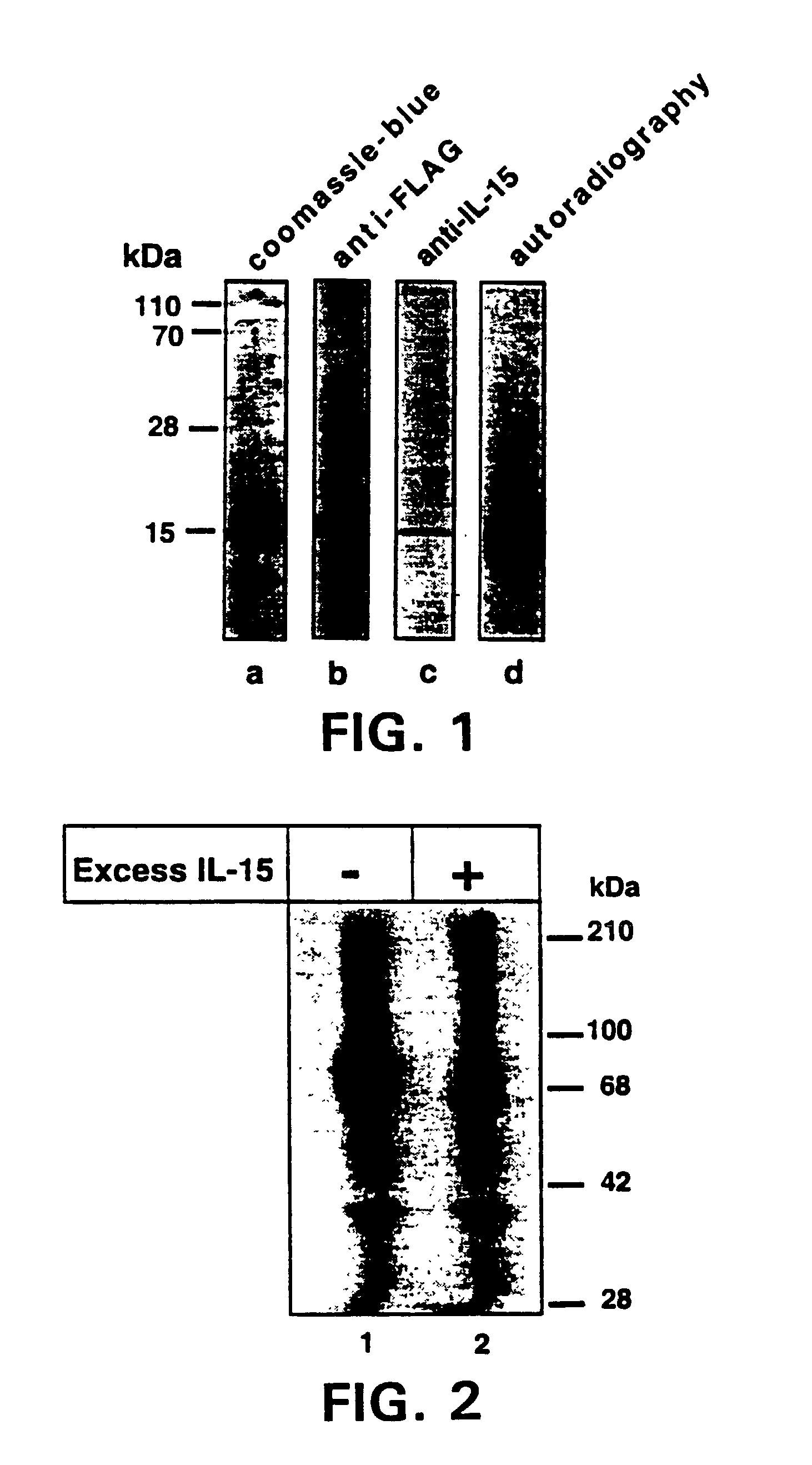
InactiveUS6998476B2Effectively competeEasy to modifyNervous disorderBacteriaWhite blood cellInterleukin 15
Disclosure herein are mutant IL-15 polypeptides and methods for using these polypeptides to modulate the immune response in a patient.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Interleukin-15 gene modified natural killing cell strain and its preparation method
ActiveCN1493687ASmall doseIncrease lethalityVector-based foreign material introductionForeign genetic material cellsWhite blood cellCulture mediums
An interleukin-15 (IL-15) gene modified natural killing cell strain for immunotherapy of tumor is prepared through inserting the cDNA coding region of IL-15 in pcDNA3 eucaryotic expression carrier, configuring secretion-type recombinant eucaryotic expression carrier pc DNA3 / IL-15, using liposom to transfecte cell NK-92, adding Geneticin (G418) to screening culture medium, screening, limited dilution to cloned cells, detecting activity, choosing positive cell clones, and amplification.
Owner:UNIV OF SCI & TECH OF CHINA +1
PEG interleukin 15
ActiveCN102145178AExtended dosing cyclePeptide/protein ingredientsPharmaceutical non-active ingredientsSmall peptideConnexon
The invention relates to a PEG interleukin 15 conjugate, namely PEG interleukin 15. PEG and interleukin 15 are connected on an N terminal amino group through or not through small peptide connexon to obtain the uniform PEG interleukin 15 conjugate. The activity of the original interleukin 15 can be at least reserved in vivo, and the PEG interleukin 15 conjugate has longer half-life period and average time to peak.
Owner:BEIJING KAWIN TECH SHARE HLDG
Use of interleukin-15
InactiveUS20070134718A1Promote generationPromotes persistencePeptide/protein ingredientsMicrobiological testing/measurementInterleukin 15Ige reactivity
The invention relates to the use of IL-15 or active variants thereof and / or IL-15 activity enhancing compounds for the manufacture of a pharmaceutical composition for manipulating memory cells of the immune system, such as manipulating viability and / or responsiveness of said memory cells. The IL-15 activity enhancing compound is for example lipopolysaccharide (LPS). The invention further relates to the use of IL-15 inhibiting or eliminating compounds for the manufacture of a pharmaceutical composition for manipulating memory cells of the immune system. Such inhibiting or eliminating compounds are for example anti-IL-15 antibodies, anti-IL-15Rα antibodies, fragments of these antibodies, e.g. the Fab or F(ab′)2 fragment, soluble IL-15Rα, fusion proteins consisting of soluble IL-15Rα, and Fc fragment, compounds, e.g. peptides, binding and / or inhibiting functional IL-15 receptor, IL-15 antisense oligonucleotides.
Owner:GROOTEN JOHAN ADRIAAN MARC +2
Interfusion protein of human interleukin 15 and Fe
ActiveCN100334112CPeptide/protein ingredientsAntibody ingredientsWhite blood cellImmunoglobulin Fc Fragments
A human interleukin 15-Fc fusion protein composed of interleukin 15 and the Fc fragment of human immunoglobulin, which are linked via joining peptide, the nucleic acid ofr coding it, the expression carrier containing said nucleic acid, its composite medicine for preventing and treating microbial infection and its preparing process are disclosed.
Owner:上海海欣生物技术有限公司
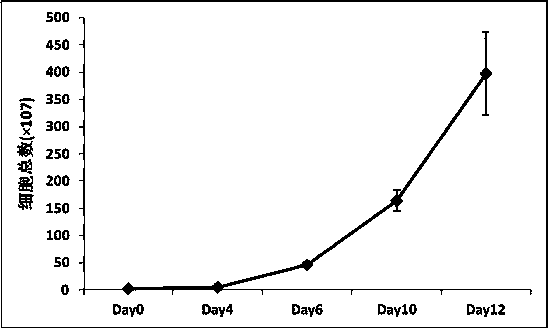
Culture medium efficiently amplifying autologous NK cells and cultural method
The invention relates to a culture medium efficiently amplifying autologous NK cells and a culture method. The culture medium internally contains interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNFalpha) and CD3-antibody, can efficiently amplify and activate NK cells so as to obtain a large quantity of highly active immune cells with majority of NK cells to be transferred back to human bodies, and has remarkable curative effect in anti-tumor, anti-virus infection and immune adjustment related diseases. The culture medium efficiently amplifying autologous NK cells comprises the culture medium for cultivating cells and added factors added into the culture medium for cultivating cells, is used for cultivating and amplifying a large quantity of autologous activated NK cells, and is characterized in that the added factor comprises interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNF alpha) and CD3-antibody.
Owner:康思葆(北京)生物技术有限公司
Adoptive transfer of cd8+ t cell clones derived from central memory cells
ActiveUS20140356398A1Mammal material medical ingredientsArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Interleukin 15 as selectable marker for gene transfer in lymphocytes
ActiveUS9487800B2Opening possibilityBiocideAntibody mimetics/scaffoldsADAMTS ProteinsAntigen receptors
The present invention relates to the use of interleukin-15 (IL-15) as selectable marker for gene transfer, preferably of at least one gene of therapeutic interest, into a mammalian cell or cell line, in particular a human cell or cell line. The present invention furthermore relates to transgenic mammalian cells or cell lines expressing IL-15 as selectable marker and co-expressing at least one protein of interest encoded by at least one gene of interest, which is preferably a protein of therapeutic interest. The present invention is in particular suitable for chimeric antigen receptors (CARs) as the gene or protein of interest and their expression in lymphocytes. The transgenic mammalian cells and cell lines are furthermore suitable for use as a medicament, in particular in the treatment of cancer and in immunotherapy, such as adoptive, target-cell specific immunotherapy.
Owner:CHEMOTHERAPEUTISCHES FORSCHUNGINSTITUT GEORG SPEYER HAUS
Drug and tumor whole-cell vaccine for treating or preventing tumor, and preparation methods and applications of drug and whole-cell vaccine
InactiveCN102343086AImprove immunityAmplify specific immune responseAntibody medical ingredientsAntineoplastic agentsAntineoplastic ImmunotherapeuticWhite blood cell
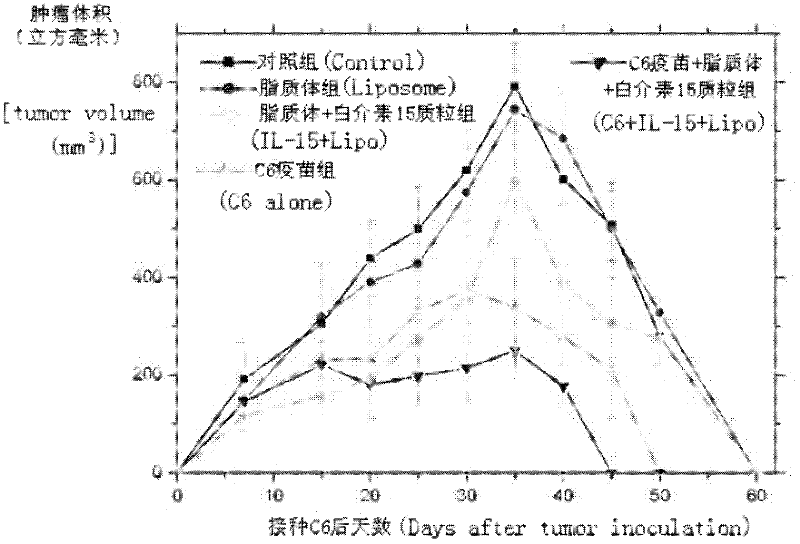
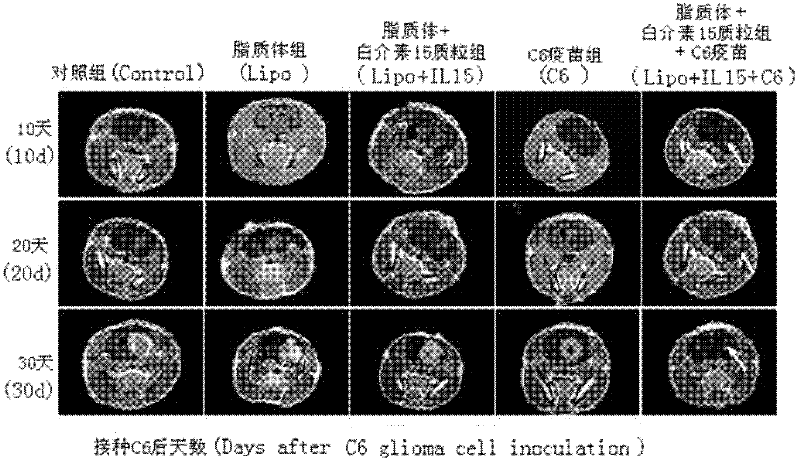
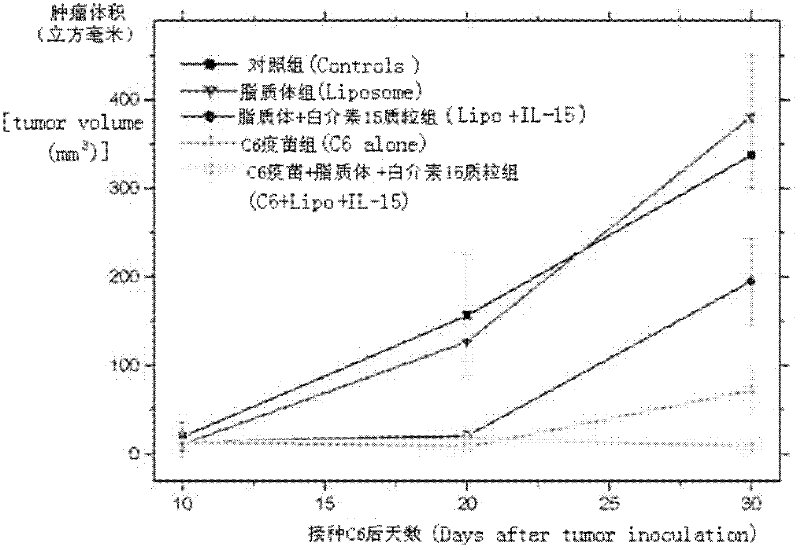
The invention relates to a drug and a tumor whole-cell vaccine for treating or preventing tumor, and preparation methods and applications of the drug and the whole-cell vaccine, and belongs to the fields of bioengineering and biological immunology. The invention provides a drug with good effect for treating or preventing tumor and a tumor whole-cell vaccine. The drug comprises the tumor whole-cell vaccine, a cation liposome, and recombinant plasmid for encoding interleukin-15 gene. The drug has the dual actions of tumor prevention immunization and anti-tumor immune treatment, and has durable action and obvious tumor prevention or treatment effect.
Owner:SICHUAN UNIV
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS8778615B2Easy to adaptMicrobiological testing/measurementAntibody ingredientsInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Expansion of lymphocytes with a cytokine composition for active cellular immunotherapy
The present invention relates to a composition for expanding lymphocytes comprising at least two types of cytokines selected from interleukin 2 (IL-2), interleukin 15 (IL-15) and interleukin 21 (IL-21). It further relates to a Method of preparing a population of clinically relevant lymphocytes, comprising the steps of: obtaining a body sample from a mammal in particular a tissue sample or body liquid sample, comprising at least one lymphocyte and optionally separating the cells in the body sample, culturing the body sample in-vitroto expand and / or stimulate lymphocytes in the sample wherein the culturing comprises using IL-2, IL-15 and / or IL-21, and optionally determining the presence of clinically relevant lymphocyte in the cultured sample.The present invention also relates to an immunotherapy and the population of clinically relevant lymphocytes.
Owner:保利比奥斯博特有限责任公司
Modified natural killer cells that express IL15 and uses thereof
ActiveUS10428305B2Peptide/protein ingredientsMammal material medical ingredientsNatural killer cellNatural killer T cell
The present invention provides, in certain aspects, a natural killer (NK) cell that expresses all or a functional portion of interleukin-15 (IL-15), and methods for producing such cells. The invention further provides methods of using a natural killer (NK) cell that expresses all or a functional portion of interleukin-15 (IL-15) to treat cancer in a subject or to enhance expansion and / or survival of NK cells.
Owner:NAT UNIV OF SINGAPORE +1
Compositions and methods for modulating gamma-c-cytokine activity
ActiveUS20120329728A1Efficient deliveryImprove in vivo stabilityCosmetic preparationsSenses disorderBinding siteAutoimmune disease
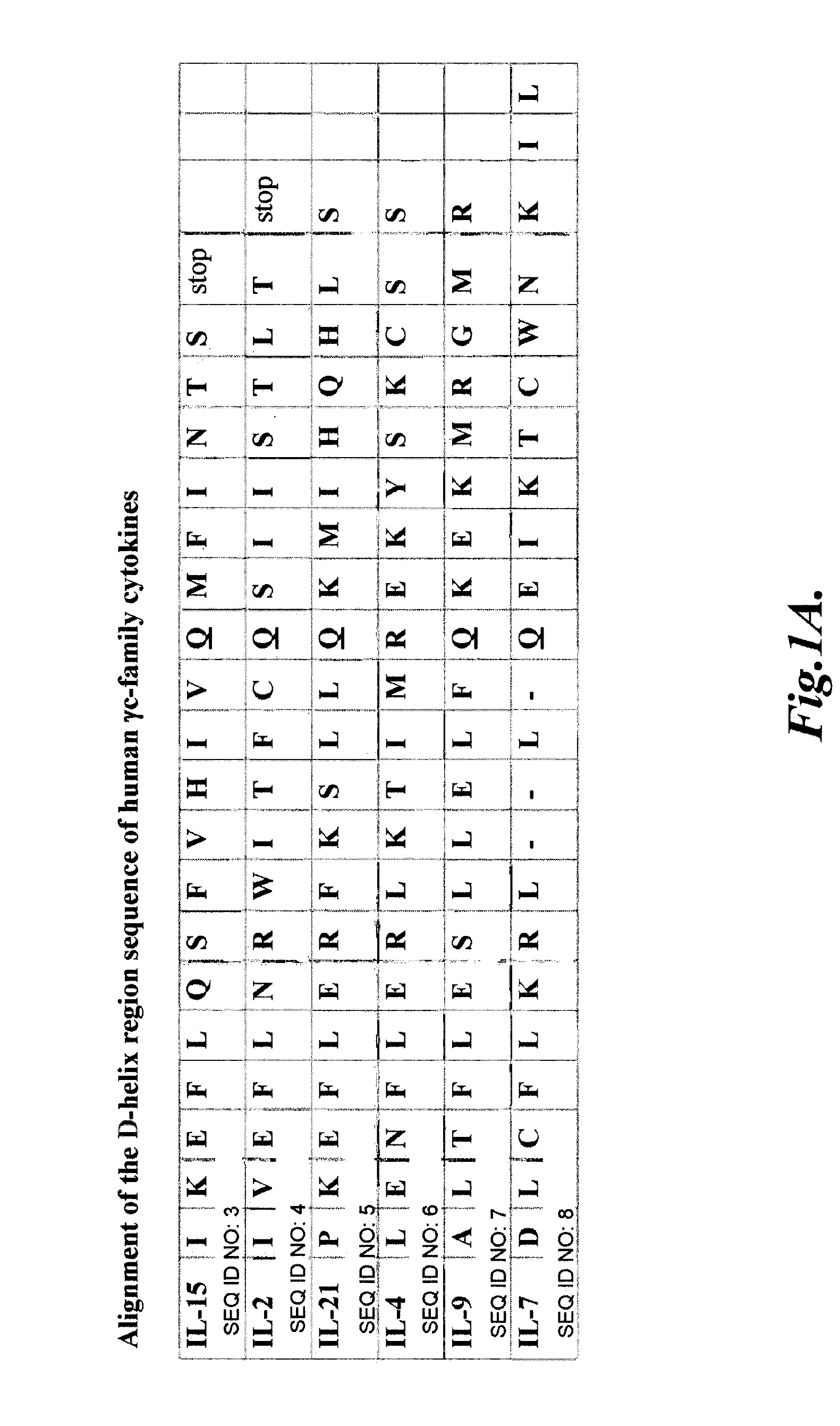
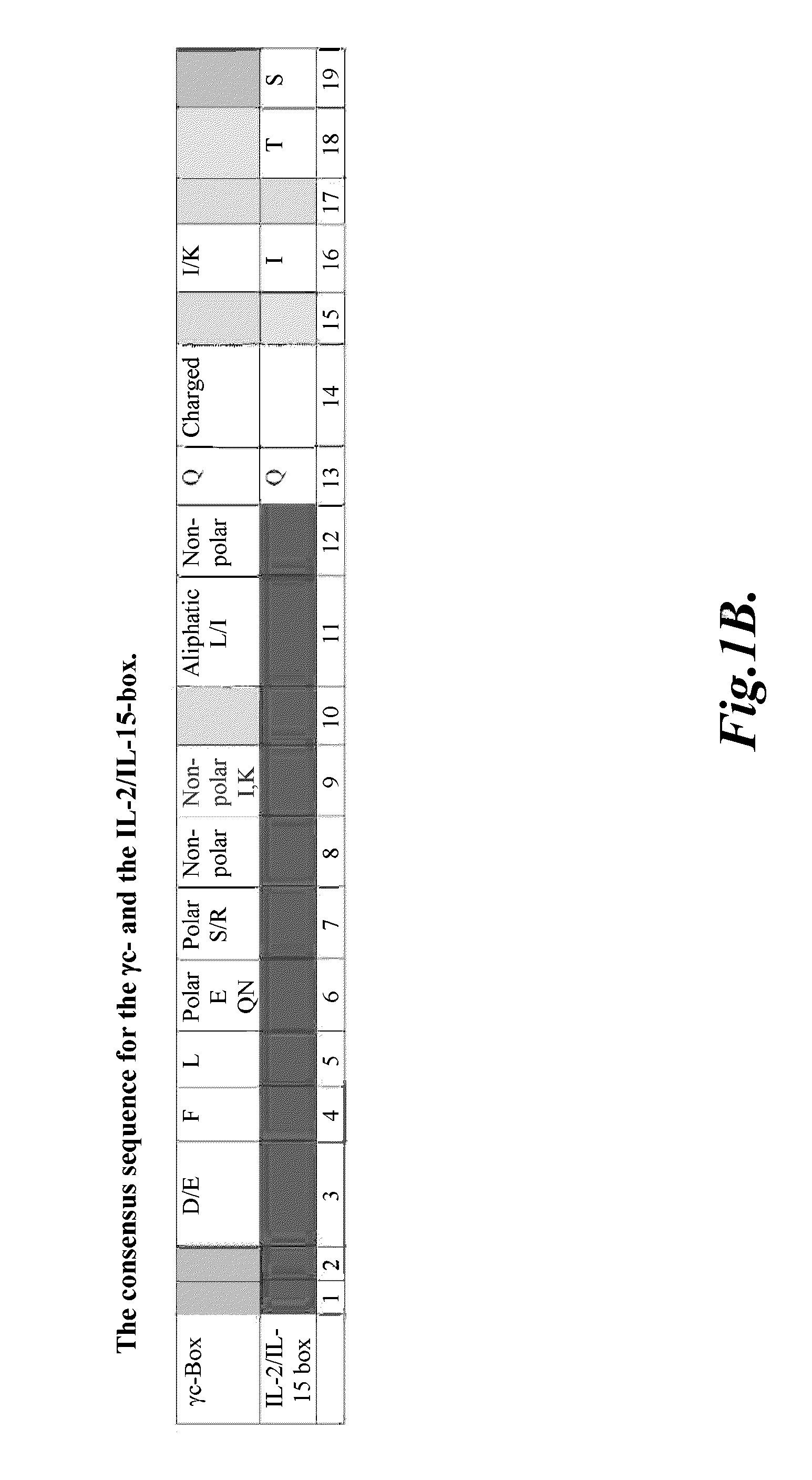
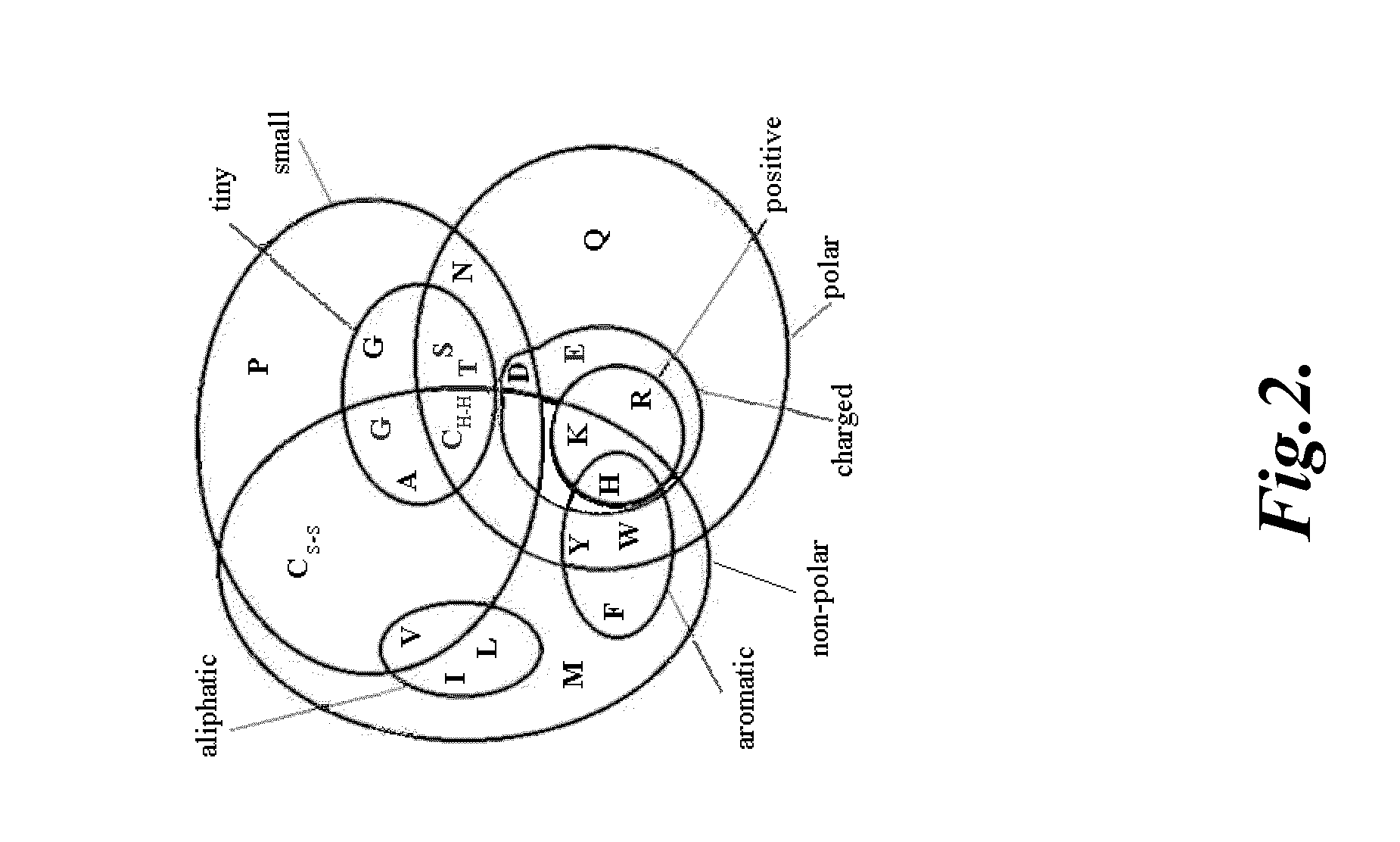
The γc-family cytokines, Interleukin-2 (IL-2), Interleukin-4 (IL-4), Interleukin-7 (IL-7), Interleukin-9 (IL-9), Interleukin-15 (IL-15), and Interleukin-21 (IL-21), are associated with important human diseases, such as leukemia, autoimmune diseases, collagen diseases, diabetes mellitus, skin diseases, degenerative neuronal diseases and graft-versus-host disease (GvHD). Thus, inhibitors of γc-cytokine activity are valuable therapeutic and cosmetic agents as well as research tools. The present embodiments relate to the design of peptide antagonists based on the consensus γc-subunit binding site to inhibit γc-cytokine activity. In several embodiments, peptide antagonists exhibit Simul-Block activity, inhibiting the activity of multiple γc-cytokine family members.
Owner:BIONIZ
Method for natural killer cell expansion
InactiveUS20180245044A1Increase heightGenetically modified cellsMammal material medical ingredientsWhite blood cellCell culture media
The present invention provides a method for in-vitro culturing and expanding natural killer (NK) cells in a cell culture medium comprising a population of NK cells, the method comprising a) adding an effective concentration of interleukin-21 (IL-21) at the beginning of the culturing process to said medium, b) adding repeatedly an effective concentration of interleukin-2 (IL-2) and / or interleukin-15 (IL-15) to said medium, and c) adding repeatedly feeder cells or membrane particles thereof to said medium, wherein said feeder cells are B cell derived which are EBV immortalized; and wherein said expansion of NK cells in said cell culture medium is maintained for at least 3 weeks.
Owner:MILTENYI BIOTEC B V & CO KG
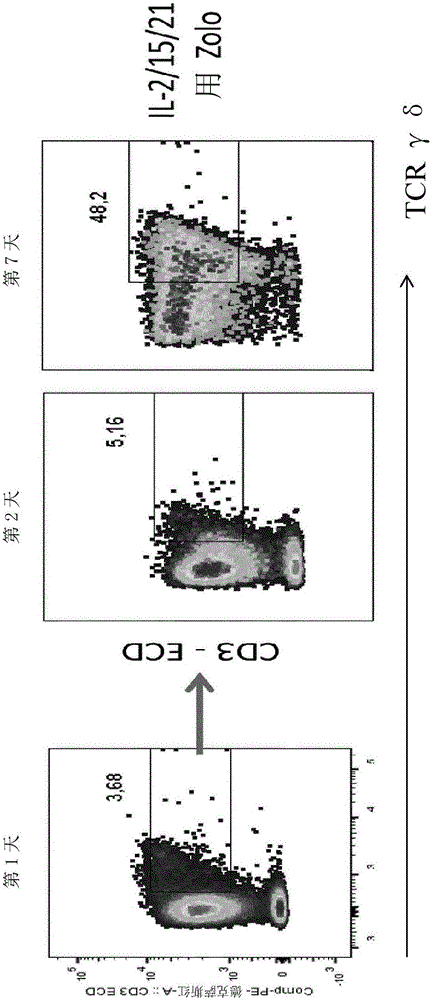
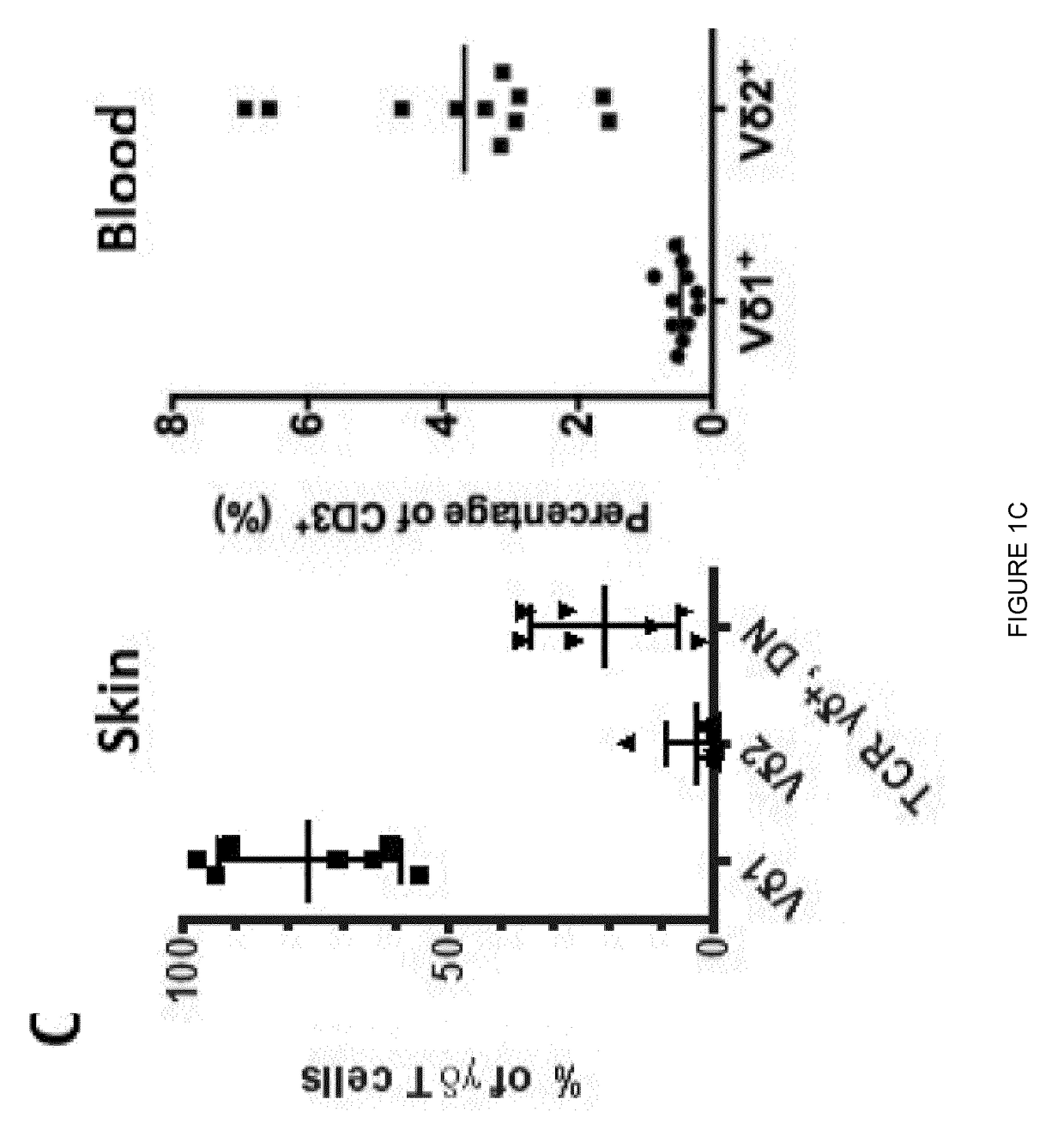
Expansion of non-haematopoietic tissue-resident gamma delta t cells and uses of these cells
ActiveUS20180312808A1Quick upgradeUnique propertyMammal material medical ingredientsAntiviralsWhite blood cellBiology
This invention relates to the expansion of non-haematopoietic tissue-resident γδ T cells in vitro by culturing lymphocytes obtained from non-haematopoietic tissue of humans or non-human animals in the presence of interleukin-2 (IL-2) and / or interleukin-15 (IL-15) and the absence of TCR activation or co-stimulation signals, without any direct contact with stromal or epithelial cells. Methods of non-haematopoietic tissue-resident γδ T cell expansion are provided, as well as populations of non-haematopoietic tissue-resident γδ T cells and uses thereof.
Owner:THE FRANCIS CRICK INST LTD
Purified interleukin-15/fc fusion protein and preparation thereof
The present invention relates to a process for purifying an interleukin-15 / Fc fusion protein from a composition, which process comprises a) applying the composition to an affinity chromatography column and eluting a first IL-15 / Fc eluate from the column and b) applying the eluate of step a) to an ion exchange chromatography column and eluting a second IL-15 / Fc eluate from the column; and to a purified interleukin-15 / Fc fusion protein and a composition, in particular a pharmaceutical composition, comprising such a fusion protein.
Owner:F HOFFMANN LA ROCHE & CO AG
Fusion protein prodrug taking interleukin 15 as active ingredient
ActiveCN110437339ASmall toxicityPeptide/protein ingredientsAntibody mimetics/scaffoldsBULK ACTIVE INGREDIENTStructural unit
The invention relates to a fusion protein prodrug taking interleukin 15 as an active ingredient. The prodrug is a fusion protein and includes the following structural units: (1) a first structural unit is the fragment of one or a subunit of an interleukin 15 (IL15) acceptor; (2) a second structural unit is IL15 having activity; (3) a third structural unit located on the C end of the fusion proteinis an antibody Fc fragment; (4) a connection fragment 1 is used for connecting the first, second and third structural units; (5) a fourth structural unit located on the N end of the fusion protein isthe extracellular fragment of the beta subunit of the IL15 acceptor; and (6) a connection fragment 2 is used for connecting the fourth structural unit and residual structural units.
Owner:IMMUNE TARGETING INC
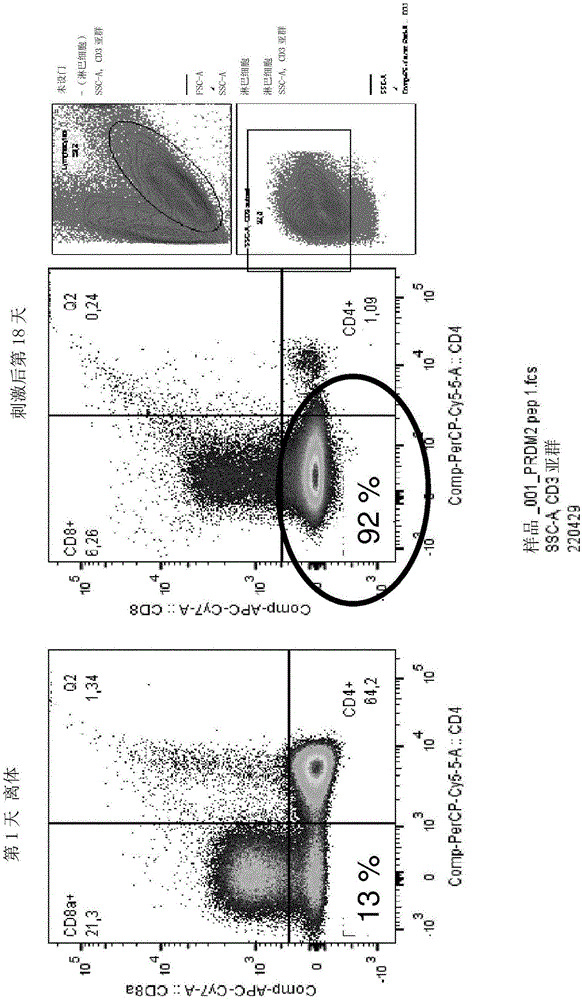
Methods of treatment with natural killer cells matched for killer immunoglobulin receptor type
ActiveUS20180353544A1Polypeptide with localisation/targeting motifOrganic active ingredientsDiseaseWhite blood cell
The present invention concerns methods of treating a disease such as leukemia in a subject by administering natural killer (NK) cells. In particular aspects, HLA-C1-licensed KIR2DL2 / 3 and KIR2DS2 NK cells are administered to a subject with an HLA-C genotype either homozygous or heterozygous for the C1 allele, or HLA-C2 licensed cells are administered to a subject with an HLA-C genotype homozygous for the C2 allele. In further aspects, the NK cells are genetically modified to express a chimeric antigen receptor and interleukin 15.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Interleukin-15 compositions and uses thereof
InactiveUS10350270B2Improve bioavailabilityPromote circulationCompound screeningNervous disorderHalf-lifeWhite blood cell
Owner:ARMO BIOSCI
Genetically modified t lymphocytes
ActiveUS20190151364A1Improve efficiencyWeight moreAntipyreticGenetically modified cellsWhite blood cellT lymphocyte
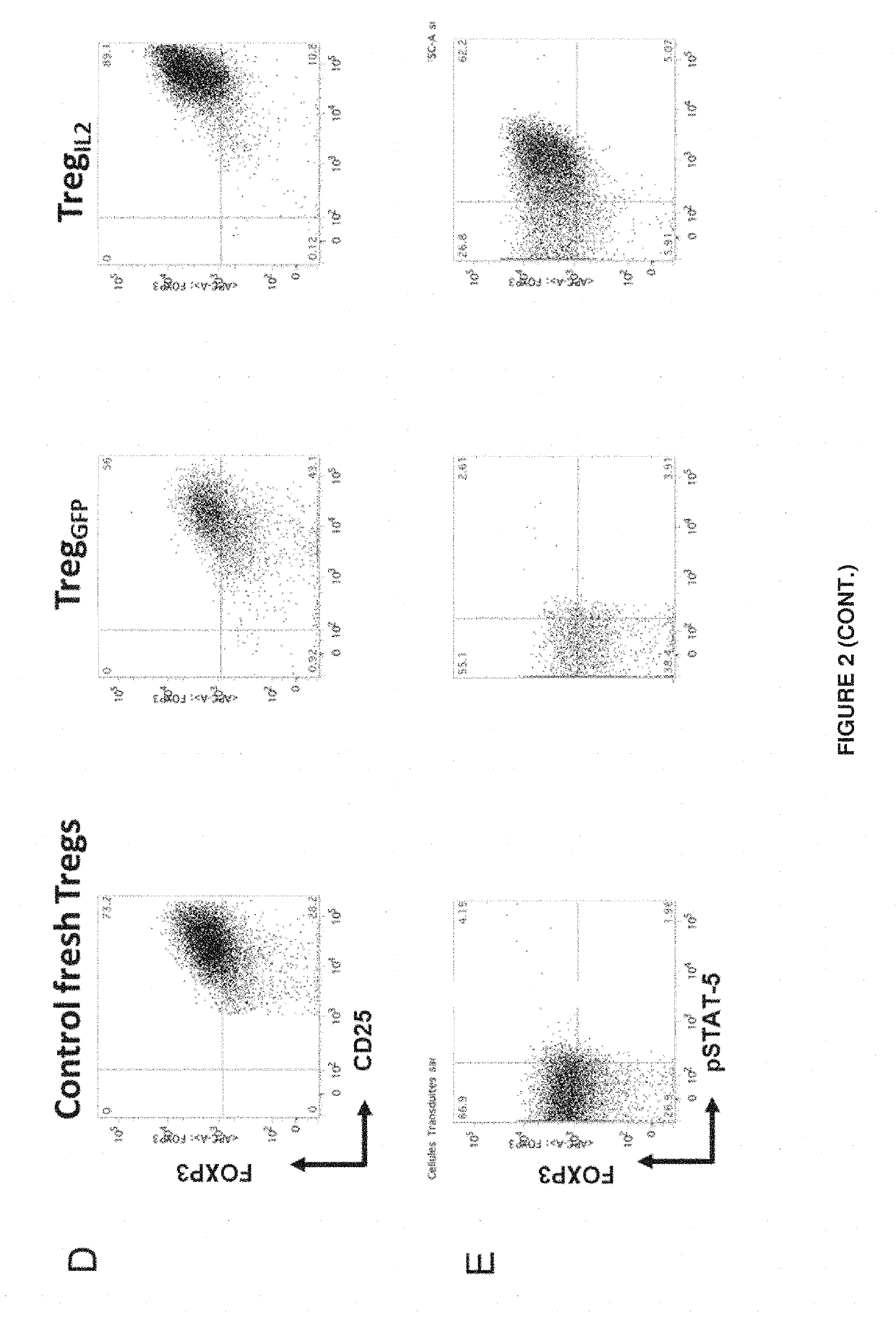
The invention relates to a composition comprising regulatory T (Treg) cells or effector T cells (Teff) which stably express an interleukin selected from the group consisting of interleukin-2 (IL-2) or interleukin-15 (IL-15).
Owner:KLATZMANN DAVID
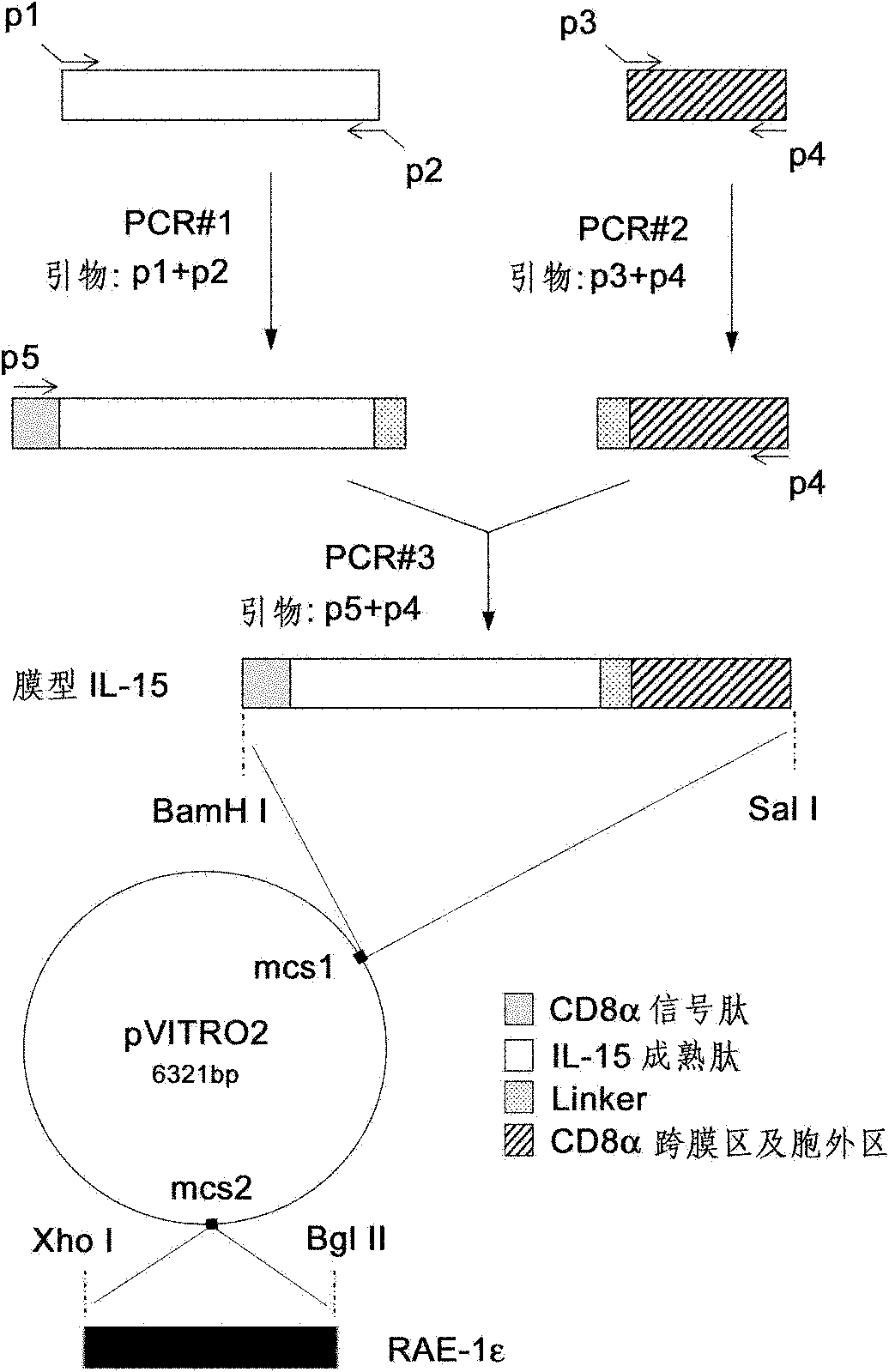
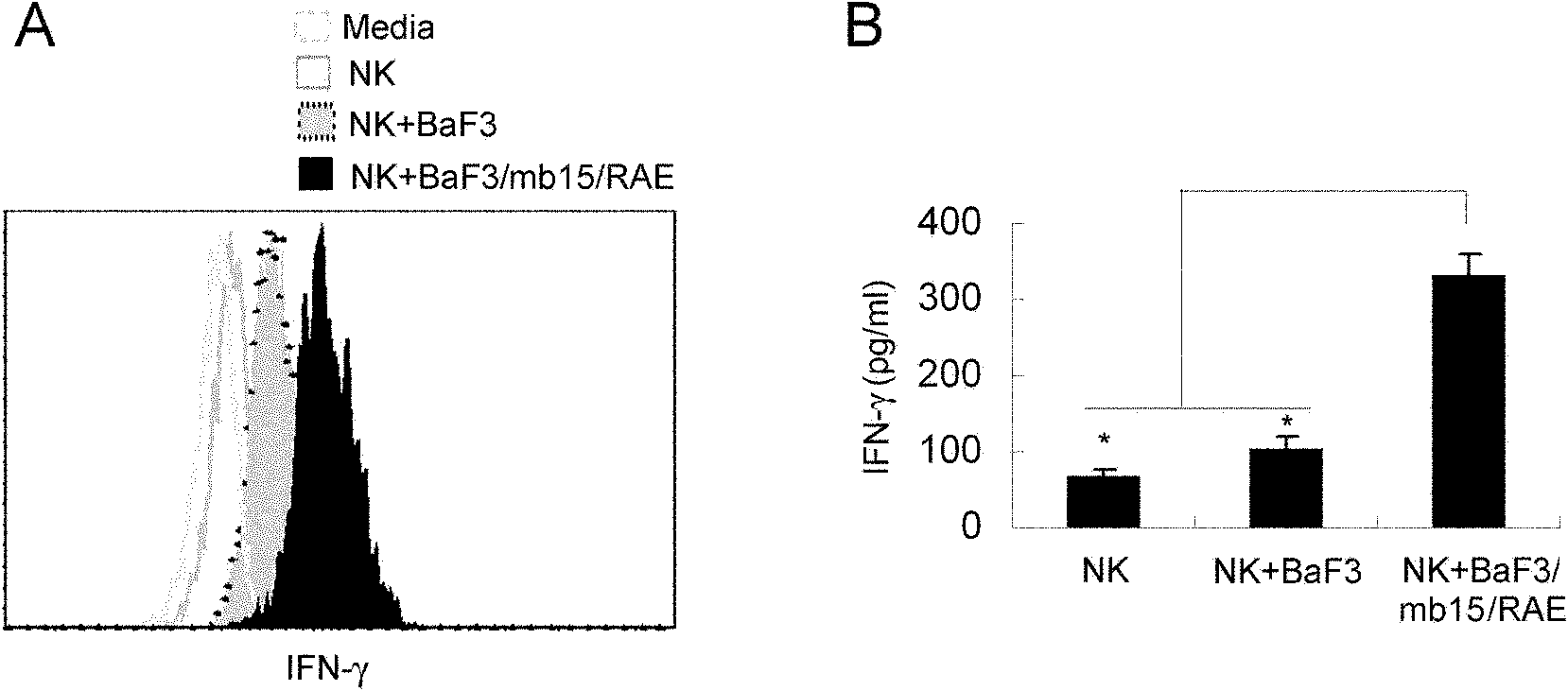
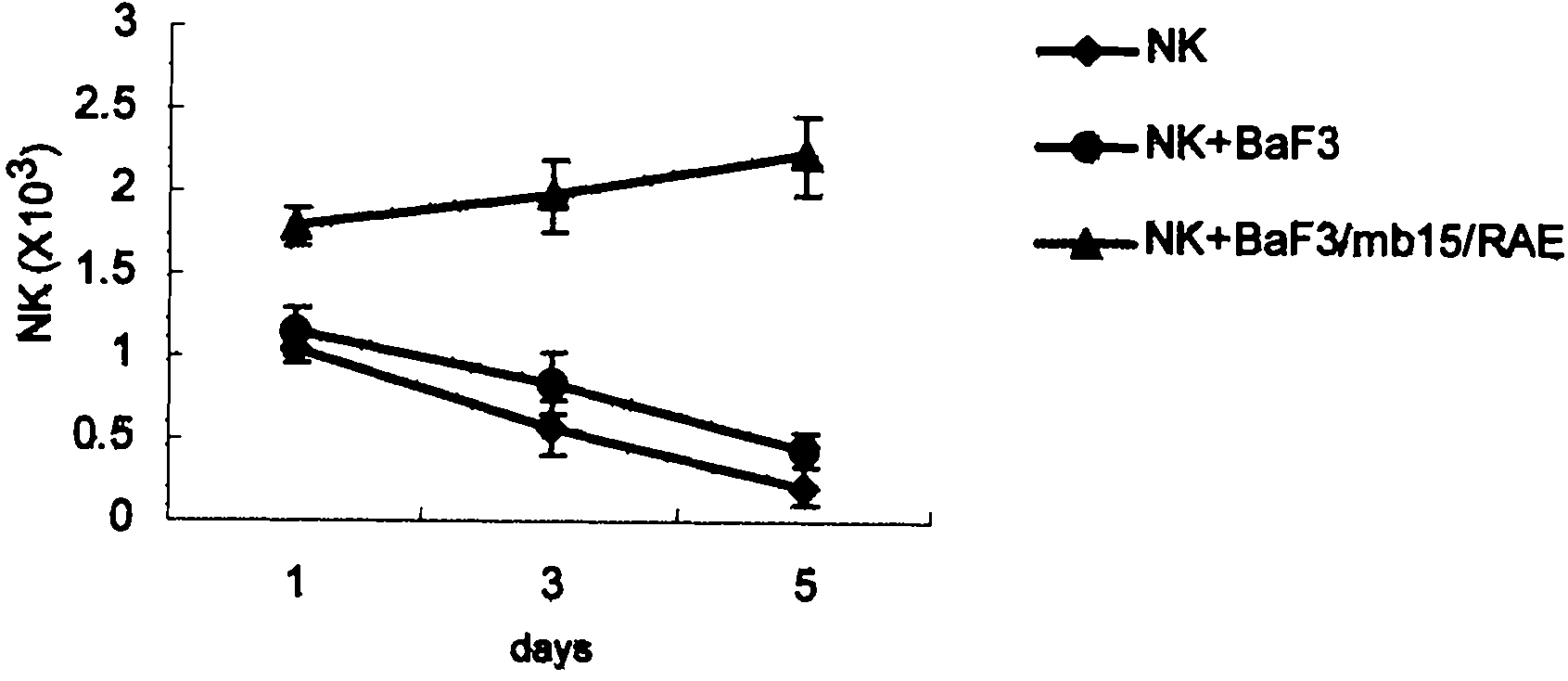
Genetically modified cell of coexpression mouse membranous type interleukin 15 and retinoic acid early transcript 1 epsilon (Rae-1 epsilon) and preparation method thereof
InactiveCN101988049AImprove targetingHigh enrichment rateFermentationVector-based foreign material introductionDendritic cellWhite blood cell
The invention relates to a genetically modified cell of coexpression mouse membranous type interleukin 15 and retinoic acid early transcript 1 epsilon (Rae-1 epsilon) and a preparation method thereof. The genetically modified cell is a BaF3 cell which can stabilize the genetically modified cell of the coexpression membranous type IL-15 and Rae-1 epsilon protein. The genetically modified cell can be prepared by a method comprising the following steps: amplifying a mouse Rae-1 epsilon gene and a mouse membrane expression type IL-15 gene by a polymerase chain reaction (PCR) technology; respectively inserting the amplified mouse Rae-1 epsilon gene and the amplified mouse membrane expression type IL-15 gene into two polyclone sites of an eukaryotic expression vector pVITRO2-mcs to acquire a recombined vector; transfecting the recombined vector into a mouse tumor cell strain BaF3; and acquiring the genetically modified cell by antibiotics screening and flow cytometry sorting. The genetically modified cell can provide IFN-gamma producing killer dendritic cells (IKDC) with triple stimulating signals as an instrument for efficiently amplifying and activating the IKDC in vitro. In addition, the genetically modified cell can also be used for antineoplastic immunotherapy research.
Owner:YANGZHOU UNIV
Interleukin-15 fusion protein for tumor targeting treatment
ActiveCN106380521AImprove anti-tumor efficacyOvercoming the short half-life problemPeptide/protein ingredientsAntibody mimetics/scaffoldsTumor targetTumor targeting
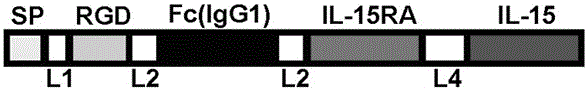
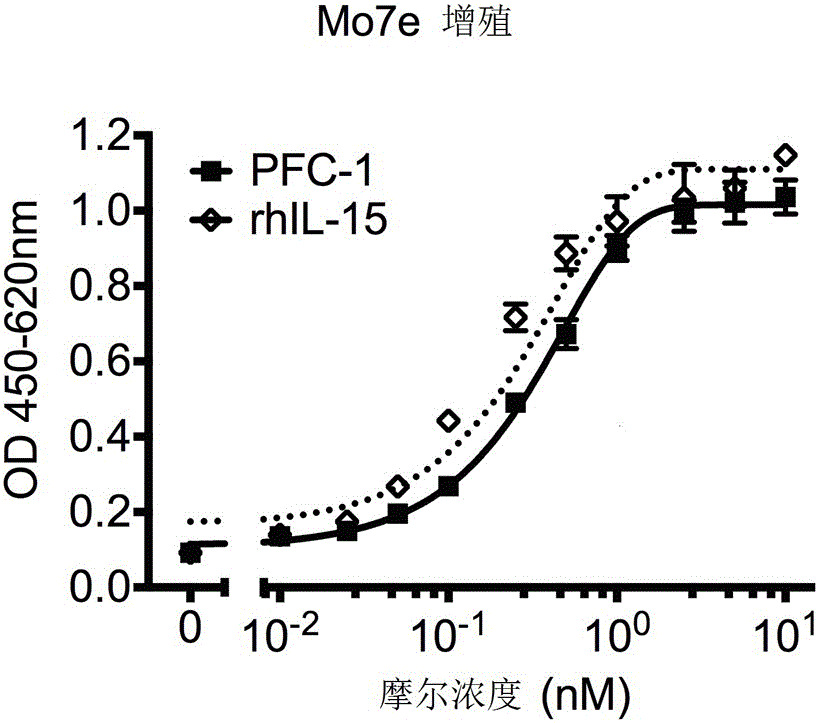
The invention provides a tumor targeting fusion protein at least comprising (i) an IL-15 polypeptide, an IL-15 polypeptide variant, or a functional fragment thereof, (ii) an IL-15Ra polypeptide, an IL-15Ra polypeptide variant, or a functional fragment thereof, (iii) an Fc structural domain, an Fc variant, or a functional fragment thereof, and (iv) an RGD polypeptide or a variant thereof. The arraying sequence of the components of the fusion protein is preferably the RGD polypeptide-the Fc structural domain-the IL-15 polypeptide-the IL-15Ra polypeptide. On one hand, the tumor targeting fusion protein can enhance the anti-tumor efficacy of the IL-15, and on the other hand, the tumor targeting fusion protein overcomes the problem of short half-life of the IL-15, also can target to a tumor site, and targetedly acts on tumor cells. In addition, the tumor targeting protein can be efficiently expressed and purified, also has efficient antitumor activity, and has the potential to become an anti-tumor therapeutic drug.
Owner:BJ BIOSCIENCE INC
Human Vgamma9Vdelta2T cell proliferation method and culture medium
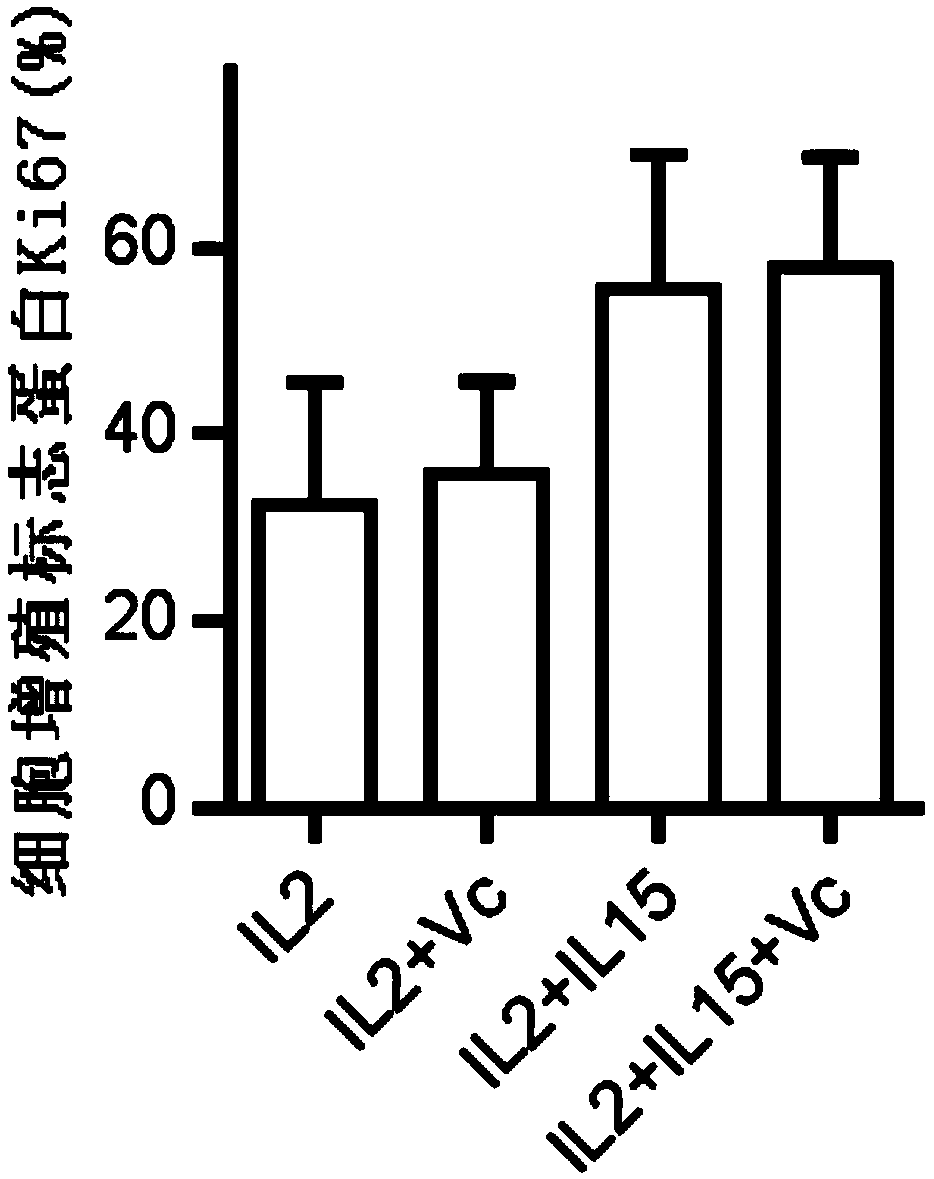
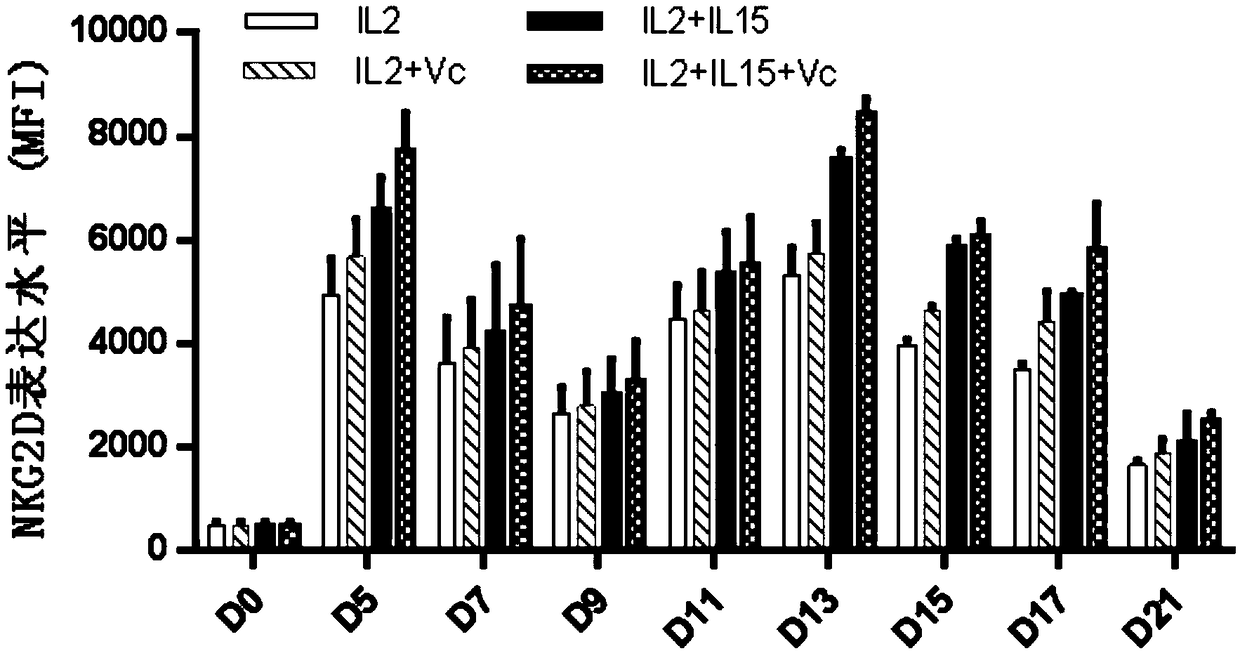
ActiveCN109337870AProliferation efficiency is fastShorten the expansion culture cycleCulture processMammal material medical ingredientsVitamin CCell survival
The invention discloses a human Vgamma9Vdelta2T cell proliferation method and a culture medium. Interleukin-15 and vitamin C are added in the proliferation culture process of human Vgamma9Vdelta2T cells, compared with a traditional proliferation method, the method can improve the proliferation efficiency and purity of the human Vgamma9Vdelta2T cells, and the cultured human Vgamma9Vdelta2T cells which have higher anti-apoptosis capacity and longer cell survival time at the same time have higher anti-apoptosis capacity, longer cell survival time and higher expression level of key killing molecules NKG2D and thus have high killing capacity for tumor cells.
Owner:GUANGDONG JIDE KANGMIN BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com