Interfusion protein of human interleukin 15 and Fe
A technology of interleukin and fusion protein, applied in the field of genetically engineered human interleukin-15 and Fc fusion protein, which can solve the problems of small molecular weight, limited biological activity, and short half-life of natural IL-15
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Cloning of human IL-15 functional gene and Fc fragment gene of human IgG4
[0049] 1. Cloning of IL-15 functional gene:
[0050] Adherent mononuclear cells were isolated from the peripheral blood of normal people, and after being stimulated by adding LPS for 4 hours, the total RNA was extracted by one-step method of guanidine isothiocyanate, the first strand of cDNA was synthesized by AMV reverse transcriptase, and PCR was carried out separately using it as a template To amplify the wild-type human mature IL-15 cDNA sequence, the primers used are:
[0051] Upstream primer: P1 5'AACTGGGTGAATGTAATAAG3'
[0052] Downstream primer: P2 5'AGAAGTGTTGATGAACA TTTG3'
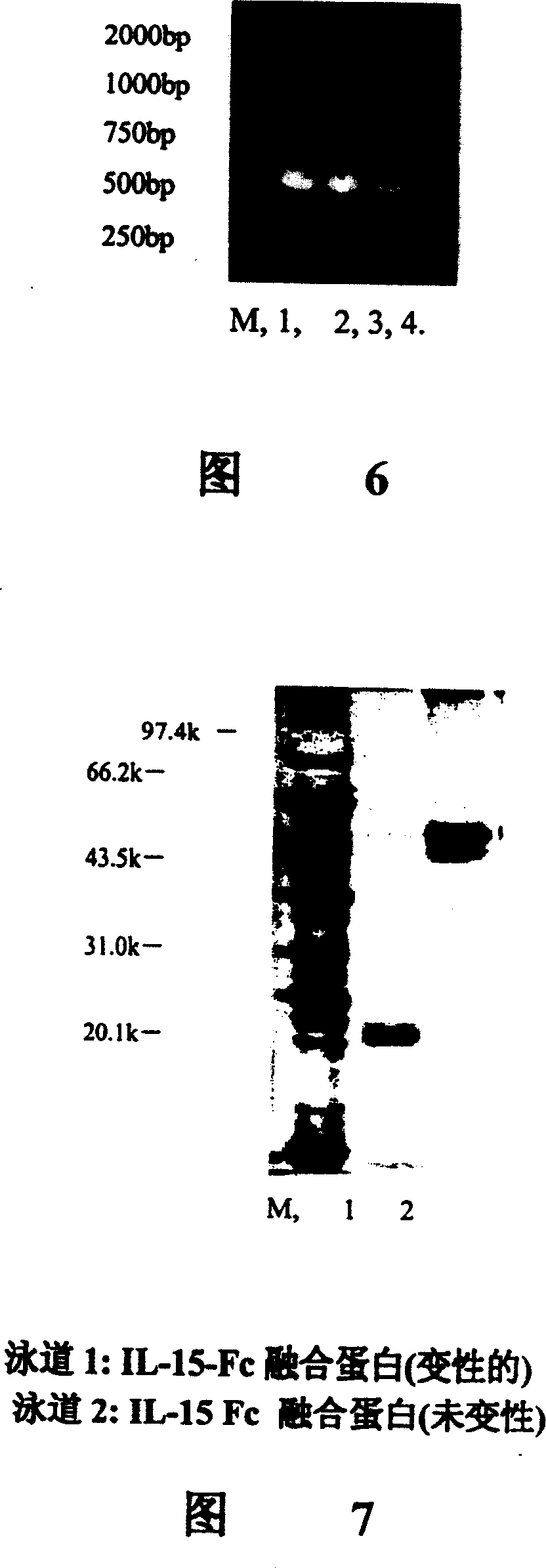
[0053] The estimated size of the PCR product of human IL-15 is 790bp, the PCR reaction volume is 50μl, the primer concentration is 0.4μM, and the amplification parameters are 94°C for 30 seconds, 51°C for 45 seconds, and 72°C for 50 seconds. After 30 cycles, the product line 2% agarose gel electrophor...
Embodiment 2
[0058] Example 2: Construction of Yeast Secretion Vector Encoding IL15-Fc Fusion Protein
[0059] Using pMD18T-IL15 and pMD18T-Fc as templates, primers were designed to amplify the IL15 and Fc fragments, and then IL15 was connected to the modified α signal peptide on the pPICK vector, while the Fc fragment was connected to the downstream region of IL15 to form a gene encoding IL15 - The carrier of the Fc fusion protein, named pPIC9K-IL15-Fc.
[0060] (a) Amplification of IL-15 fragments
[0061] For the IL15 fragment, the primer sequences used for amplification are:
[0062] Upstream primer IL15-F: -CGAGAAAAGAAACTGGGTTAACGTTATCTC-
[0063] Downstream primer IL15-RE: -CGGAATTCGGAAGTGTTGATGAACAT-
[0064] Using pMD18T-IL15 as a template, the IL15 fragment was amplified by conventional PCR method. The amplification conditions were 97°C, 2min; 94°C, 30s, 58°C, 30s, 72°C, 45s, a total of 25 cycles; then 72°C, 10min; and finally stored at 4°C. As a result, a 360bp target fragme...
Embodiment 3
[0086] Example 3: Establishment and Identification of Yeast Engineering Strains Expressing IL15-Fc Fusion Protein
[0087] Take 5-10 μg of the recombinant plasmid pPIC9K-IL15-Fc and the control plasmid pPIC9K large pumping plasmid, and after linearization by SacI digestion, transform the yeast strain GS115 by electric shock method, and use MD (glucose minimal medium) plate after transformation to screen for occurrence homologous recombination His + clone. Mix all the transformed clones with water, and take 10 5 Each yeast was spread on the G418 gradient resistance YPD plate (G418 concentrations were 0, 0.25, 0.5, 0.75, 1.0, 1.5, 1.75, 2.0, 3.0 and 4.0 mg / ml). Incubate at 30°C for 2-5 days. Pick a number of recombinant colony inoculum on each gradient resistance plate in 20ml of BMGY medium, culture in a shake flask at 30°C, grow to the bacterial cell concentration A600nm=2~6, collect the bacteria by centrifugation, and replace it with 5-10 times The volume (100ml) of BMMY ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com