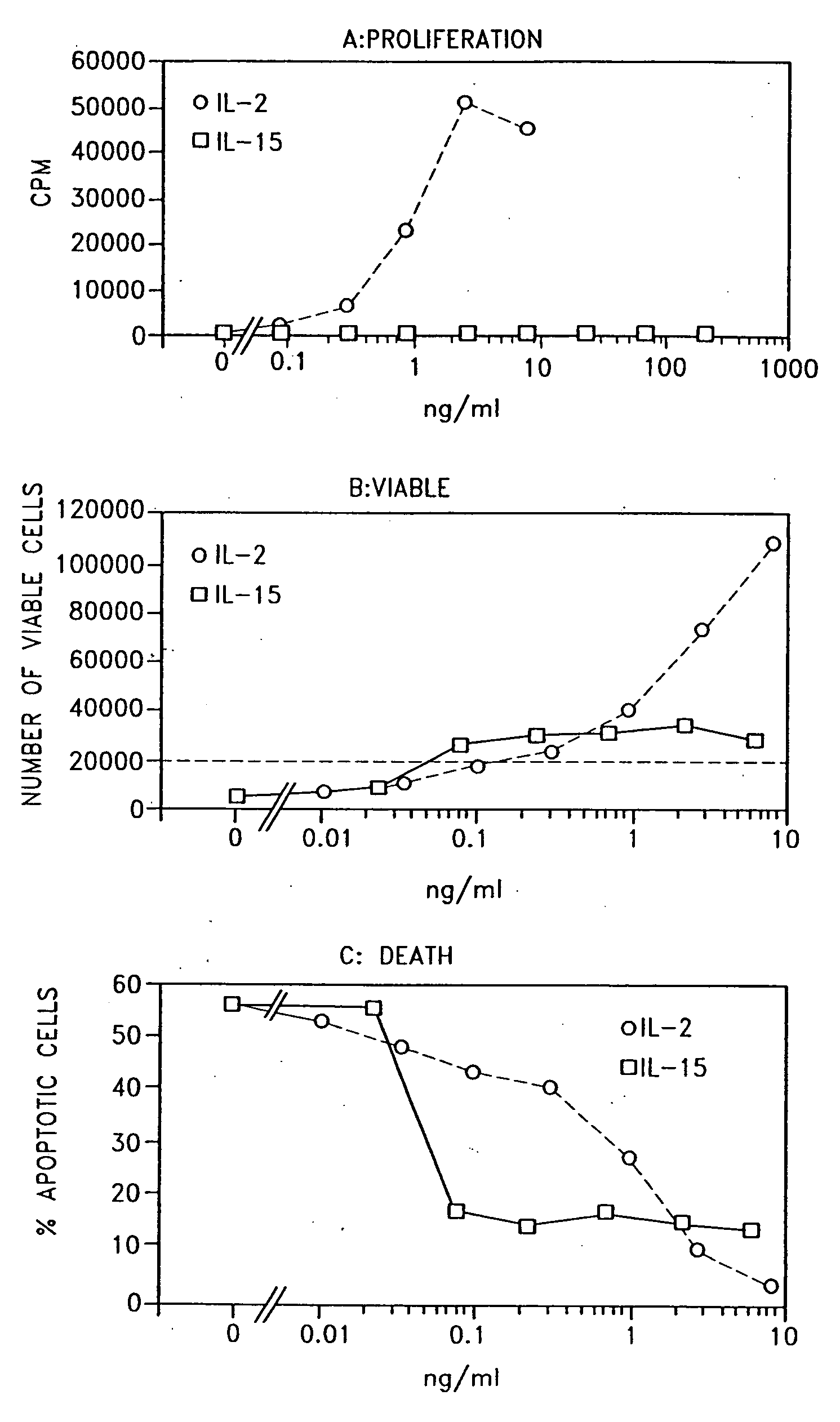
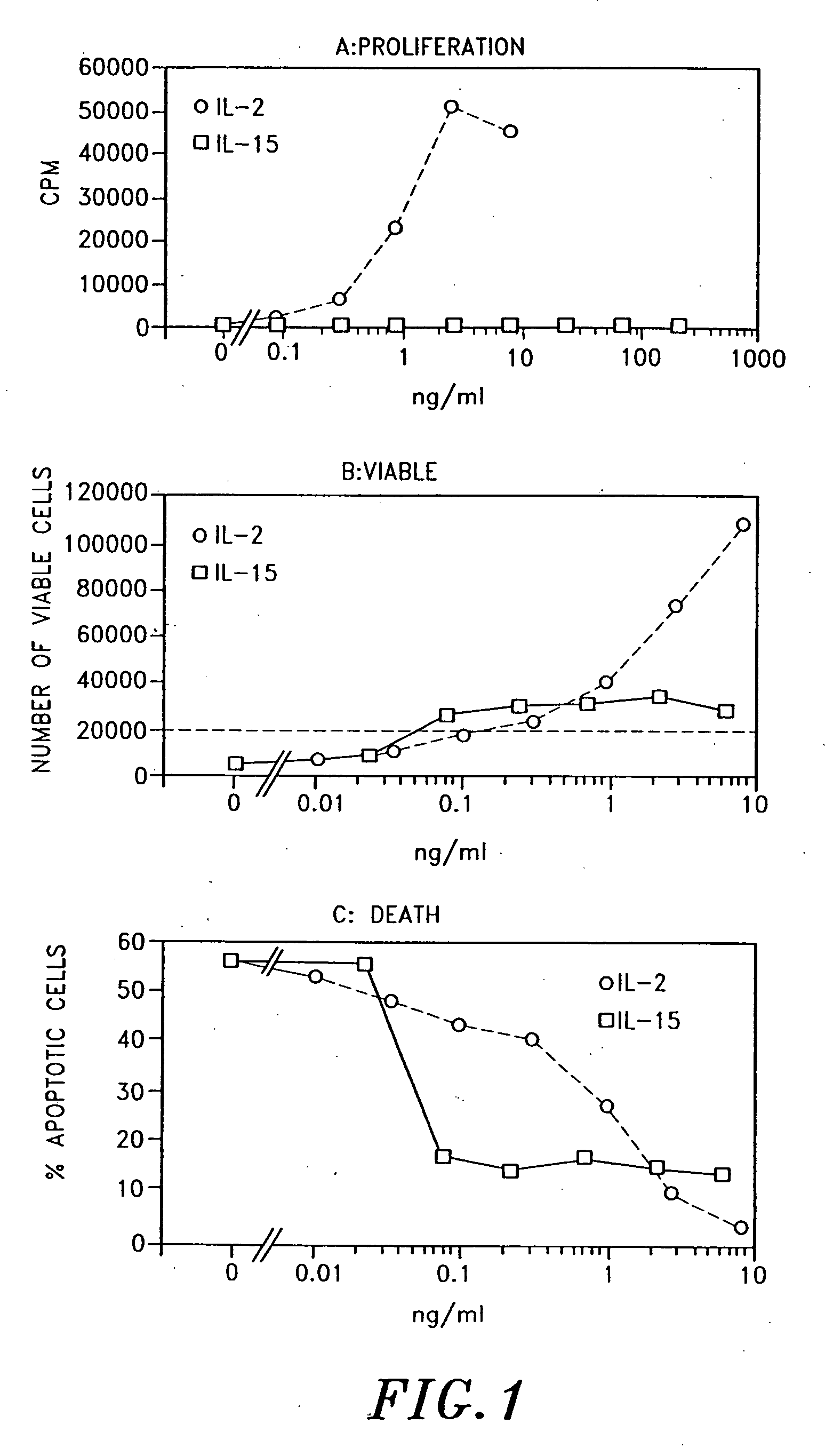
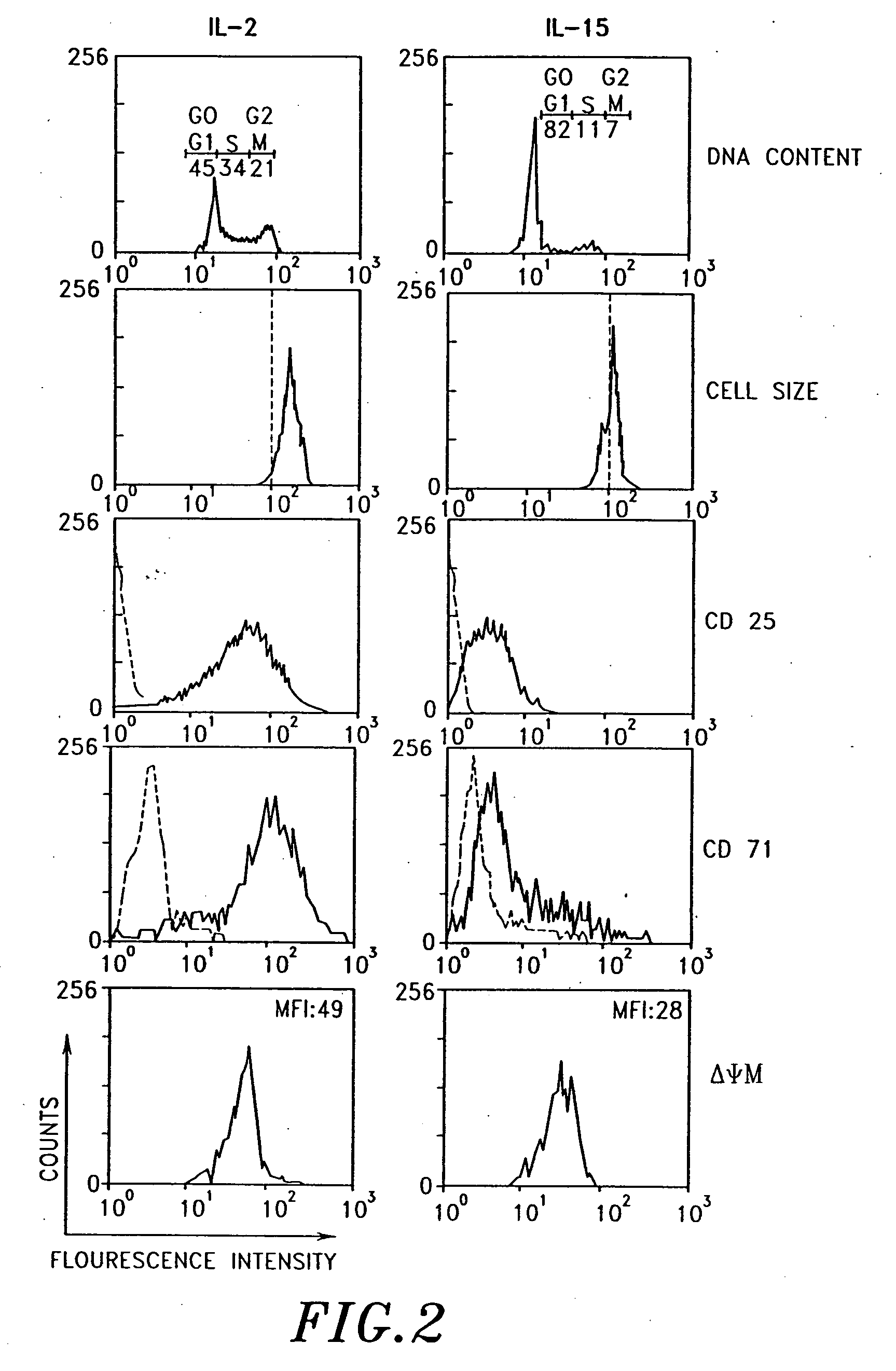
Use of interleukin-15
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
1. Materials and Methods
1.1. CD4+ T Cell Clone
[0048] The influenza A / H3 haemagglutinin (HA)-specific and H-2b restricted CD4+ murine T cell clone T-HA was developed in this laboratory by an initial immunisation of C57BI / 6 mice with 100 μg / ml bromelain cleaved haemagglutinin (BHA) and 0.5 mg / ml adjuvant (Ribi, Immunochem Research Inc., Hamilton, Mont., USA) and a second immunisation with 32 μg / ml BHA three weeks later.
[0049] 5 days after this boost immunisation lymph nodes were isolated and 3.107 cells were stimulated in vitro with 0.5 μg / ml BHA in 25 cm2 culture flasks (Nunclon, Nunc, Roskilde, Denmark). On day 4 10 U / ml mouse IL-2 (from PMA stimulated EL4.IL-2 cells) was added to the cultures.
[0050] After 2 additional biweekly restimulations with 0.5 μg / ml BHA and APC, a pool of optimally HA-reactive T-lymphocytes was obtained. These T-HA cells were maintained long term in vitro by biweekly restimulation in 25 cm2 culture flasks with 10 ng / ml BHA and 7×107 syngeneic spleen ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com