Purified interleukin-15/fc fusion protein and preparation thereof
A technology of fusion protein and protein, which is applied in the direction of interleukin, peptide preparation method, animal/human protein, etc., and can solve the problems such as the difficulty of rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
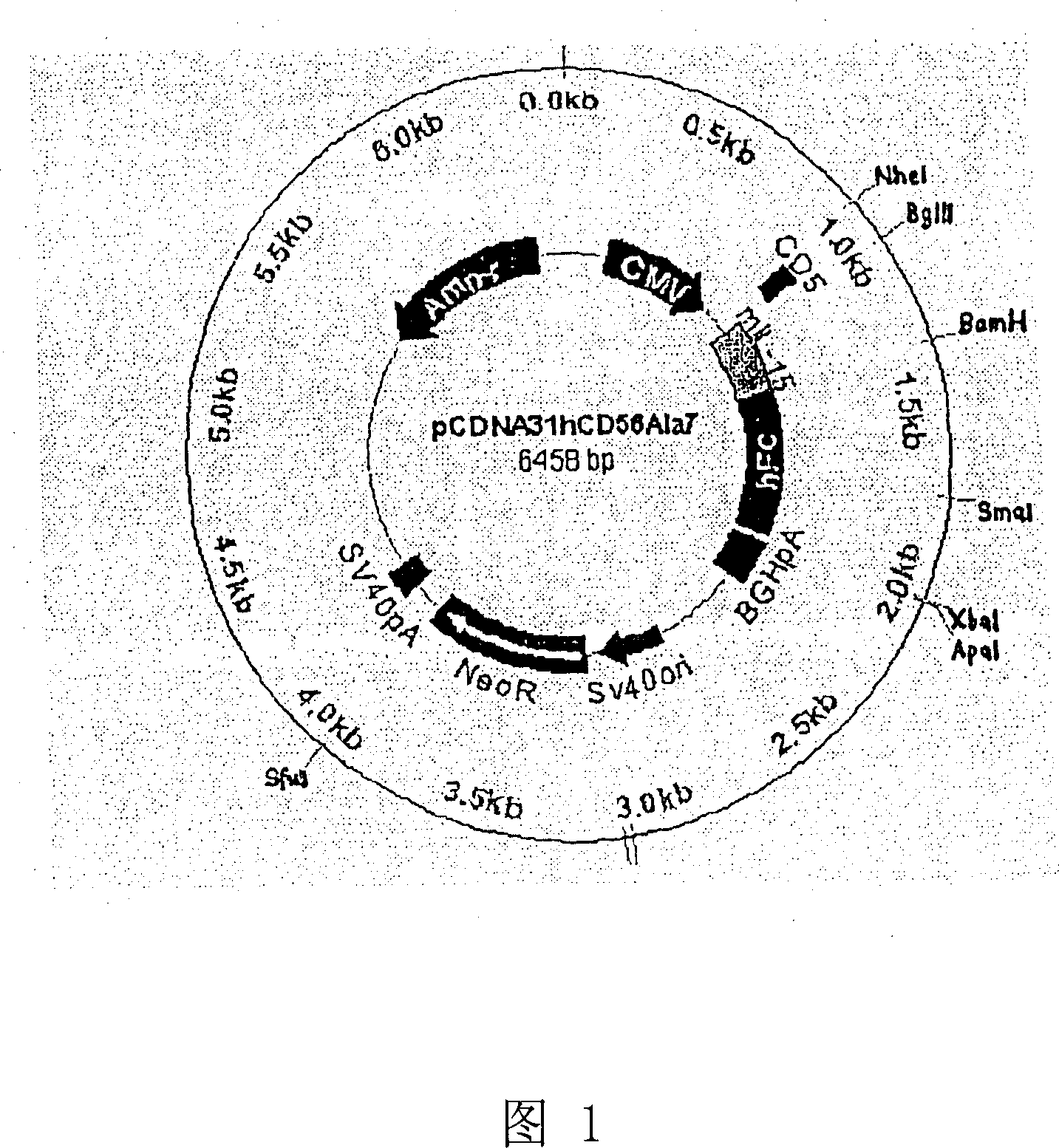
[0100] Example 1: Preparation of IL-15 / Fc in CHO-K1 cells
[0101] In order to prepare IL-15 / Fc-producing CHO-K1 cell lines, an IL-15 / Fc expression construct should be formed and tested in terms of its secretion properties, identity / integrity of the fragments it contains and suitable resistance genes optimize
[0102] a) Raw material
[0103] Human IL-15 / Fc expression construct (mutant IL-15 / human Fc) was provided by the Department of Immunology of the "Beth Israel Deaconness Medical Center" (Harvard Medical School, Boston, USA).
[0104] Oligonucleotides were obtained from NMG-Biotech (Ebersberg, Germany). Sequences of relevant signal peptides were obtained from GenBank.
[0105] Restriction enzymes (BgIII, XbaI, BamHT, SmaT, BstXI, ApaI), Lipfectamin2000, other molecular biology reagents (T4-DNA ligase, T4-polynucleotide kinase) and plasmids pSecTagA, pcDNA3.1 were obtained from Invitrogen (Karlsruhe, Germany ) or Amersham-Pharmacia (NheI, Protein A Sepharose Uppsala, Sw...
Embodiment 2
[0124] Transfection of eukaryotic cell lines (eg, CHO-K1 cells) with a plasmid containing the DNA of the desired product is a standard method for producing therapeutic proteins. However, the low productivity level of the stable cell clones thus produced is a known problem. Therefore, various methods exist for increasing the productivity of existing cell lines. In addition to attempts to increase the number of copies of the plasmid in the cell (eg via the methotrexate / DHFR system), it is also possible to modify the expression construct itself. Incorporation of introns in addition to strong promoters (such as the CMV promoter) can lead to better RNA stability and better RNA export from the nucleus, which can be carried out by the cell's splicing machinery. However, experimentation must be performed to determine which intron / transgene combination is suitable. To this end, various introns were combined with human IL-15-Fc to find out the combinatorial way to increase IL-15-Fc pr...
Embodiment 3
[0140] Embodiment 3: Purification of IL-15 / Fc fusion protein
[0141] a) purification and concentration
[0142] About 3100 liters of the supernatant from Example 2 (pMG10Ala7 plasmid) were clarified, concentrated and sterile filtered in 6 operations. This included clarification of the supernatant using a Profile Star filter (3 micron, 20 inches, Pall Corporation, East Hills, NY, USA). Subsequently, the supernatant was concentrated 10 to 15 times in total by a tangential flow filtration system (Millipore, Billerica, MA, USA) using a 2.0 square meter Biomax-30 membrane. The inlet pressure is 2-2.5 bar and the outlet pressure is 1.5 bar. After concentration, filter through a filter comprising a pre-filter (Polysep II (0.2 micron, 10 inches, Millipore, Billerica, MA, USA)) and a final filter (Durapore (0.22 micron, 10 inches, Millipore, Billerica, MA, USA)). The filter system sterile-filters the concentrate. Six different operations took 4.5 to 8 hours.
[0143] b) rProtein-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com