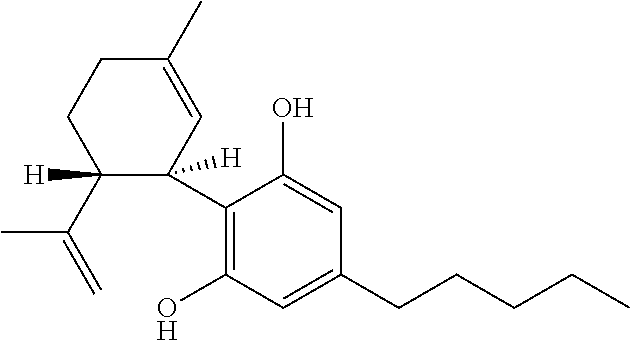
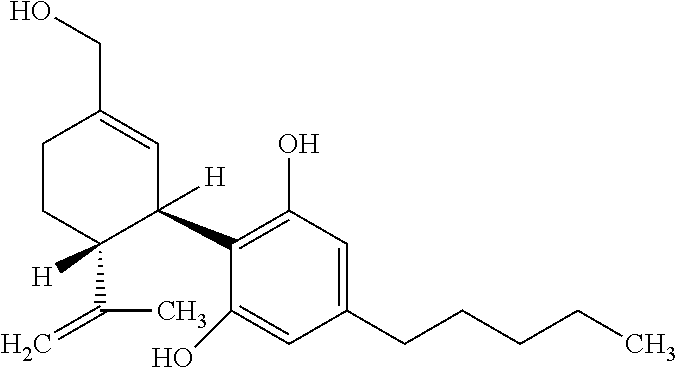
7-oh-cannabidiol (7-oh-cbd) and/or 7-oh-cannabidivarin (7-oh-cbdv) for use in the treatment of epilepsy
a technology of cannabis and cannabinoids, which is applied in the field of 7ohcannabinoids (7ohcbd) and/or 7ohcannabinoids (7ohcbdv) for use in the treatment of epilepsy, can solve the problems of difficult treatment, cognitive, behavioral and motor delays, and inability to obtain seizure freedom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
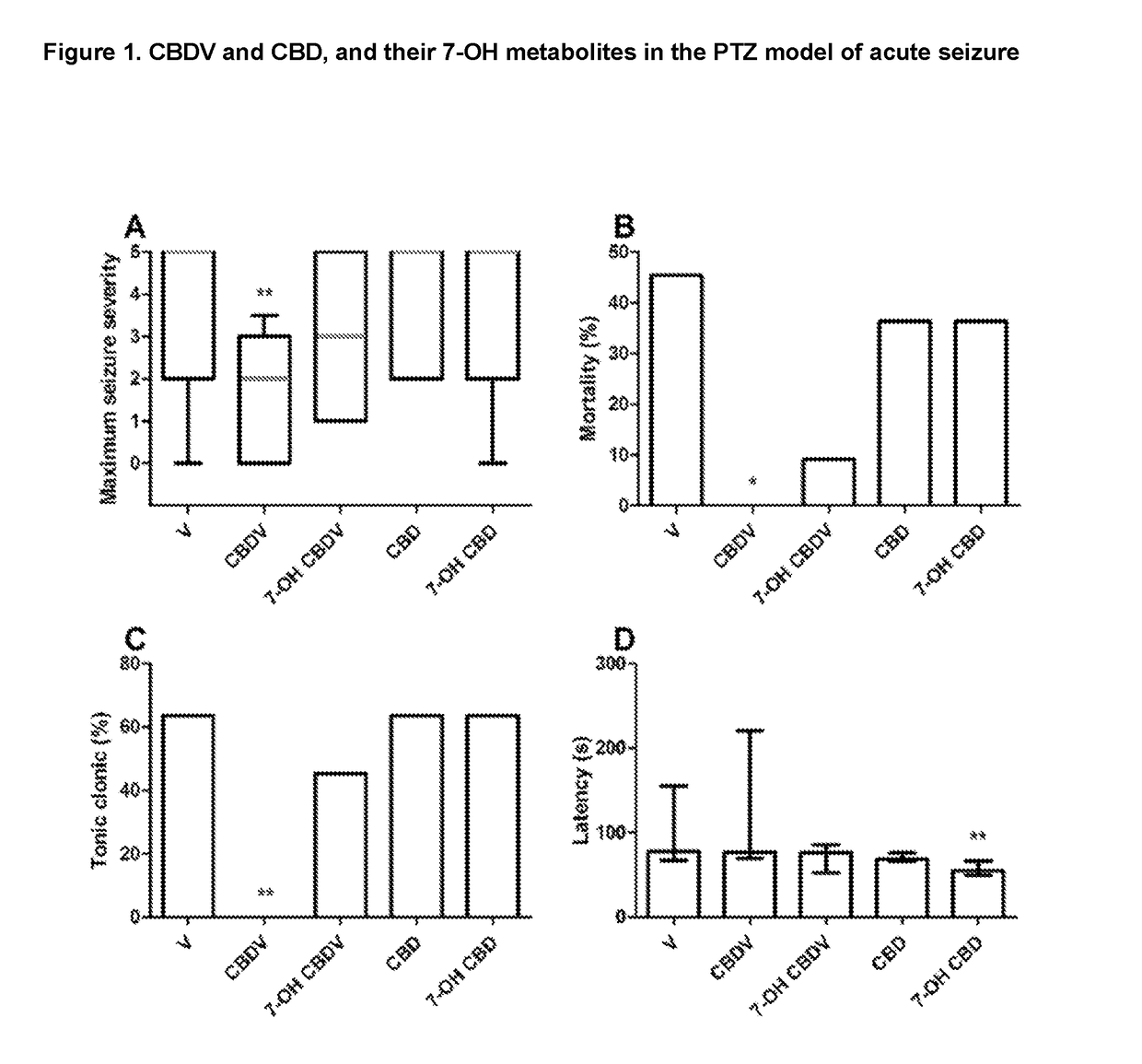
Efficacy of 7-Hydroxy Cannabidiol (7-OH-CBD) and 7-Hydroxy Cannabidivarin (7-OH-CBDV) in the Ptz Model of Seizure
Materials and Methods
Compounds:
[0054]The compounds 7-OH CBD and 7-OH-CBDV have never been tested in a model of epilepsy and as such the effects were examined at one dose level in order to determine efficacy.
[0055]The hydroxy-metabolites of CBD and CBDV were also tested against their parent cannabinoids which were used as positive controls. The table below details the doses used in this study.
CompoundDose (mg / kg)Vehicle—CBDV2007-OH-CBDV200CBD1007-OH CBD100
General Methodology for PTZ Model
Animals
[0056]Male Wistar rats (P24-29; 75-110 g) were used to assess the effects of the cannabinoids listed above on the PTZ model of generalised seizures. Animals were habituated to the test environment, cages, injection protocol and handling prior to experimentation. Animals were housed in a room at 21° C. on a 12 hour light: dark cycle (lights on 0900) in 50% humidity, with free access ...
example 2
Efficacy of 7-Hydroxy Cannabidivarin (7-OH-CBDV) in the Maximal Electroshock (Mes) Model of Seizure
Preparation of Test and Reference Compounds
[0072]The vehicle used in this study was 2:1:17 (ethanol:Cremophor:0.9% w / v NaCl). The test compound used was 7-OH-CBDV. This was made to a solution at the highest concentration; then dissolved in ethanol before combination with Cremophor and 0.9% NaCl in the proportion described above. The 7-OH-CBDV was administered intraperitoneally at a volume of 10 ml / kg body weight.
Test System
[0073]Animal Species / Strain: Mouse / ICR, Microbiological grade: SPF, Inc. Sex: male, Age (at time of testing): 5-7 weeks old, Number of animals: about 5 animals per group. Temperature: 23±2° C., Humidity: 60±10%, Light conditions: 7 AM to 7 PM for the light period, 7 PM to 7 AM for the dark period. Chow and water: Free access to CRF-1 (Oriental Yeast Co, Ltd) and tap water.
Experimental Procedures
[0074]One day before each experiment, mice were weighed and randomized in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com