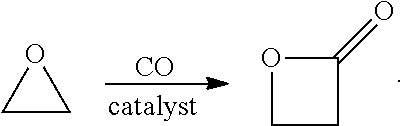Flexible chemical production platform
a flexible and chemical technology, applied in the field of chemicals, can solve the problems of not being able to easily accommodate or integrate the production of other chemicals, and achieve the effect of high reactive and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 4
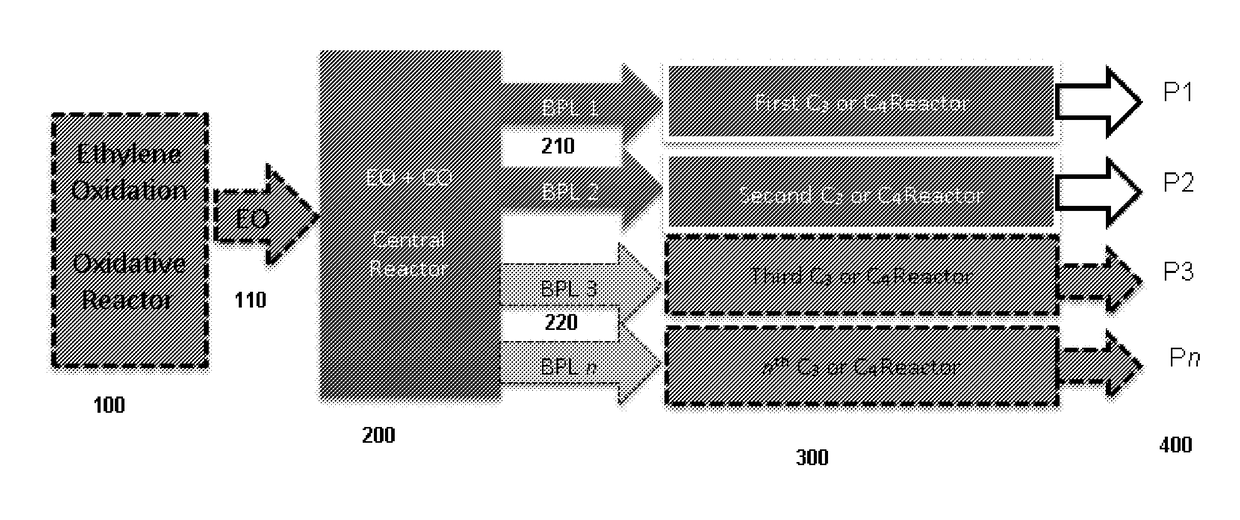
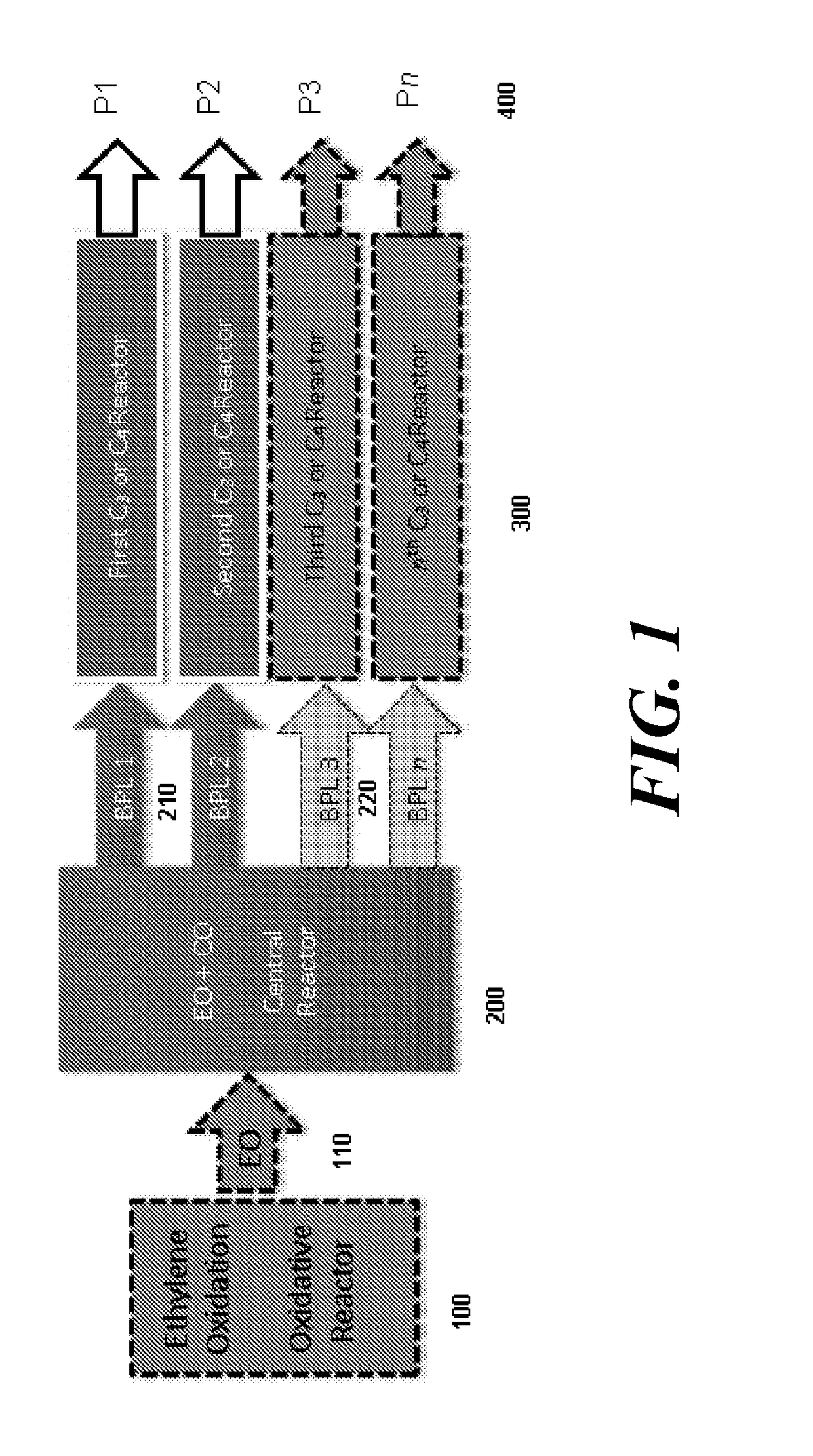
5. The system of embodiment 4, further comprising an oxidative reactor, comprising an inlet fed by ethylene, an oxidative reaction zone that converts at least some of the ethylene to EO, and an outlet which provides an outlet stream comprising the EO, which is fed to the inlet of the central reactor.
6. The system of embodiment 1, wherein the first C3 product and the second C3 product are independently selected from an α,β-unsaturated acid, an α,β-unsaturated ester, an α,β-unsaturated amide, a polymer and 1,3-propanediol (PDO).
7. The system of embodiment 6, wherein the first C3 product is polypropiolactone (PPL).
8. The system of embodiment 6, wherein the first C3 product is acrylic acid.
9. The system of embodiment 1, wherein the first C3 product is PPL, and the system further comprises a third C3 reactor, comprising an inlet fed by the outlet stream comprising PPL of the first C3 reactor, a third C3 reaction zone that converts at least some of the PPL to a third C3 product, and an ou...
embodiment 14
15. The system of embodiment 14, wherein the system produces the AA from ethylene.
16. The system of embodiment 15, comprising:[0910]an oxidative reactor, comprising an inlet fed by ethylene, an oxidative reaction zone that converts at least some of the ethylene to ethylene oxide (EO), and an outlet which provides an outlet stream comprising the EO, which is fed to an inlet of a central reactor,[0911]the central reactor, comprising the inlet fed by the outlet stream comprising the EO from the oxidative reactor and a carbon monoxide (CO) source, a central reaction zone that converts at least some of the EO to beta propiolactone (BPL), and an outlet which provides an outlet stream comprising the BPL,[0912]one or more of:[0913](i) a first C3 reactor, comprising an inlet fed by the outlet stream comprising BPL of the central reactor, a first C3 reaction zone that converts at least some of the BPL to a polypropiolactone (PPL), and an outlet which provides an outlet stream comprising the P...
embodiment 41
42. The system of embodiment 41, wherein the system is configured to produce AA at about 200 to about 800 kilotons per annum (kta).
43. The system of any one of embodiments 32 to 42, wherein the first C4 product is succinic anhydride.
44. The system of any one of embodiments 32 to 42, wherein the first C4 product is succinic anhydride, and the system further comprises:[0962]a second C4 reactor comprising:[0963]an inlet configured to receive the outlet stream comprising succinic anhydride of the first C4 reactor,[0964]a second C4 reaction zone configured to convert at least some of the succinic anhydride to a second C4 product, and[0965]an outlet configured to provide an outlet stream comprising the second C4 product.
45. The system of embodiment 44, wherein the second C4 product is succinic acid, 1,4 butanediol (BDO), tetrahydrofuran (THF) or gamma butyrolactone (GBL).
46. A system, comprising:[0966]an ethylene source;[0967]a carbon monoxide (CO) source;[0968]an alcohol source;[0969]an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com