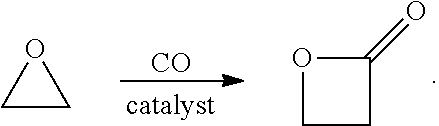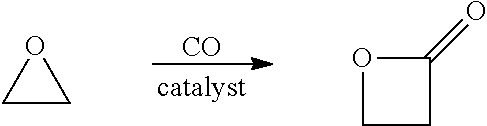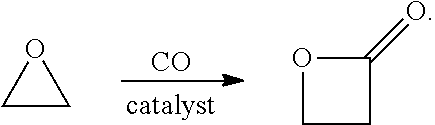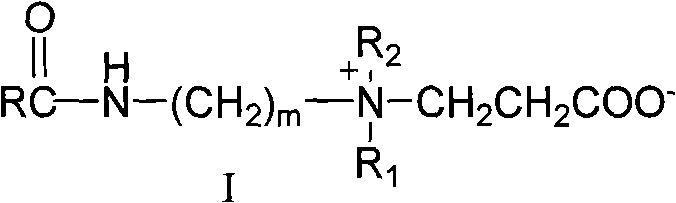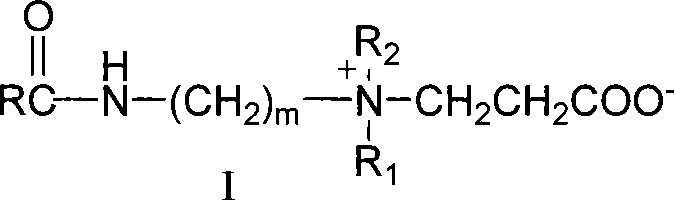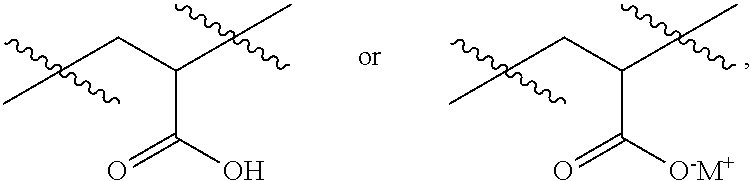Patents
Literature
70 results about "Beta-Propiolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
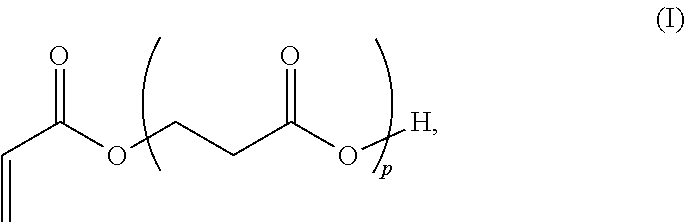
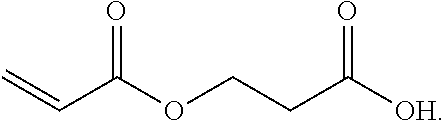
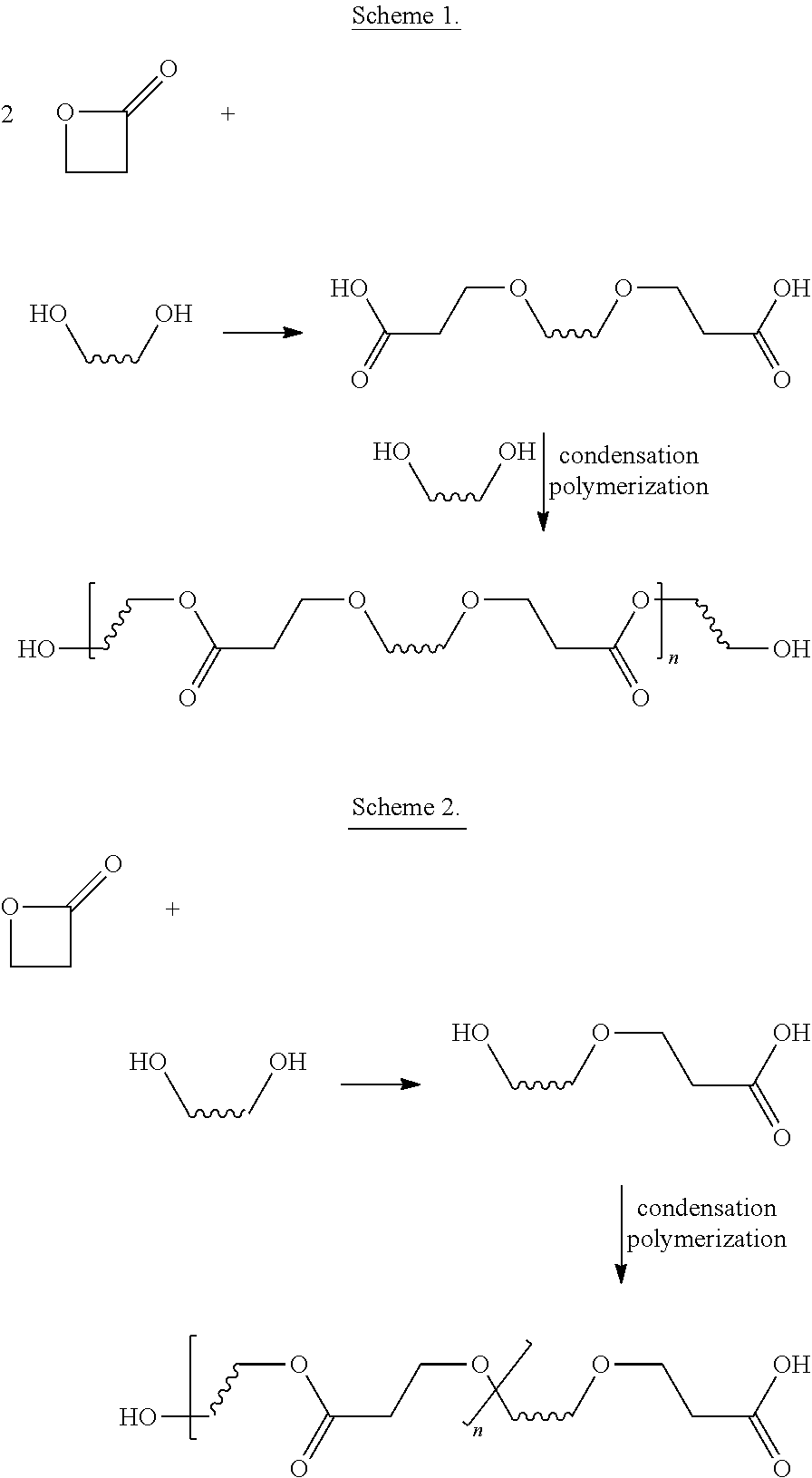
Β-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and miscible with ethanol, acetone, diethyl ether and chloroform. The word propiolactone usually refers to this compound, although it may also refer to α-propiolactone.
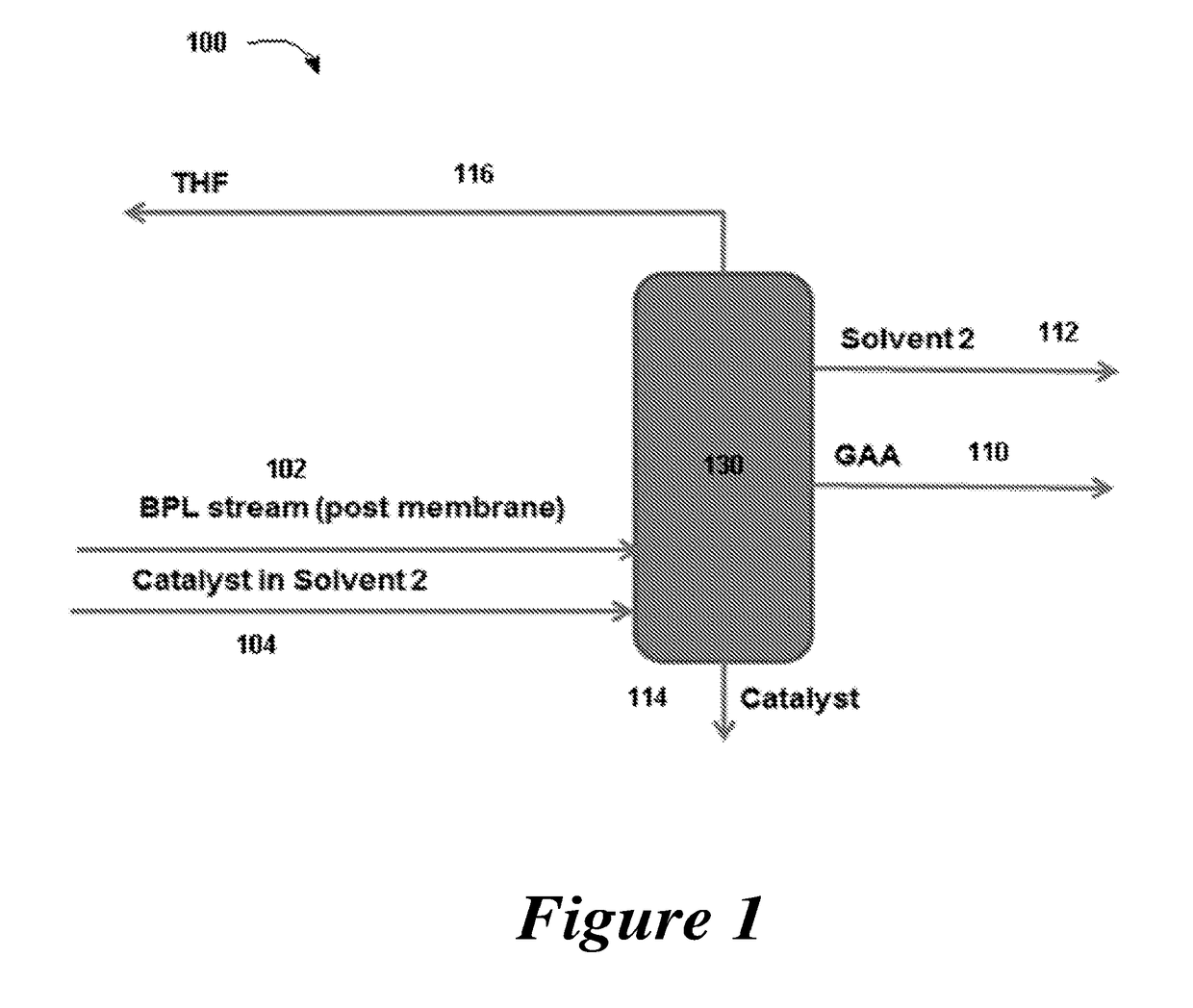
Distillation process for production of acrylic acid
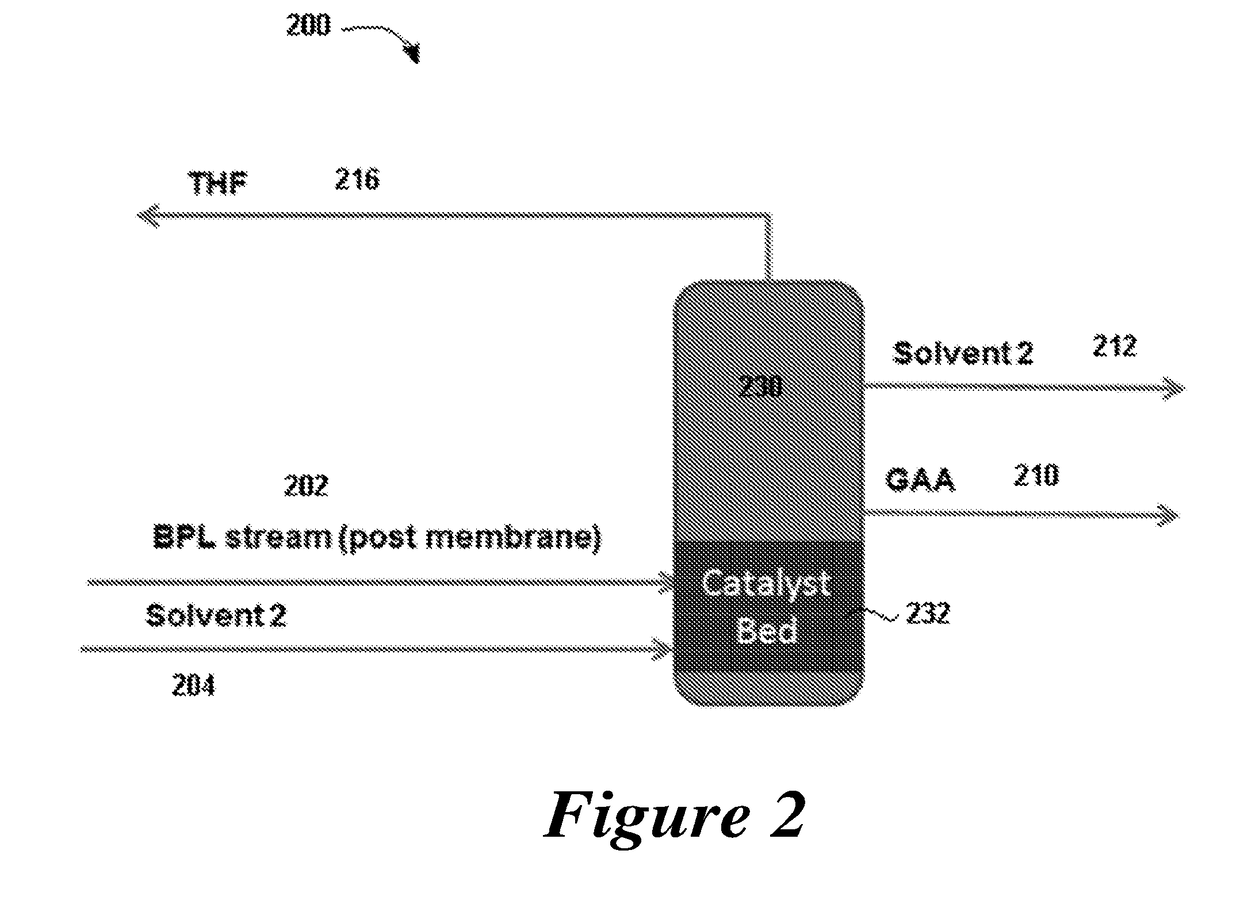
ActiveUS20180022677A1Chemical industryPreparation from carboxylic acid esters/lactonesDistillationBeta-Propiolactone
Provided are integrated processes for the conversion of beta propiolactone to acrylic acid. Systems for the production of acrylic acid are also provided.
Owner:NOVOMER INC
Process for production of acrylic acid
ActiveUS20180016219A1Promote aggregationReduce adverse effectsChemical industryPreparation from carboxylic acid esters/lactonesBiochemistryBeta-Propiolactone
Provided are integrated processes for the conversion of beta propiolactone to acrylic acid. Systems for the production of acrylic acid are also provided.
Owner:NOVOMER INC
Systems and processes for polyacrylic acid production
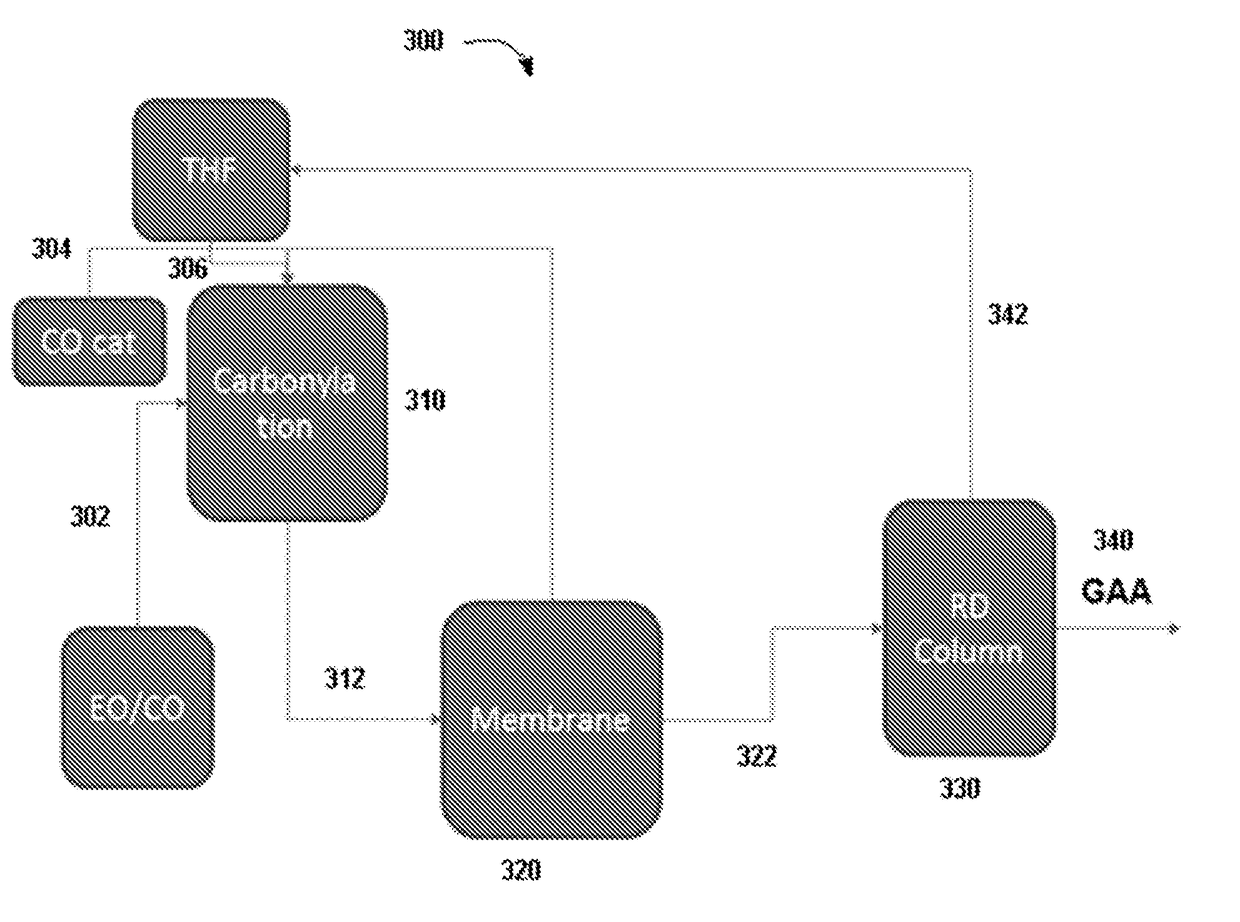
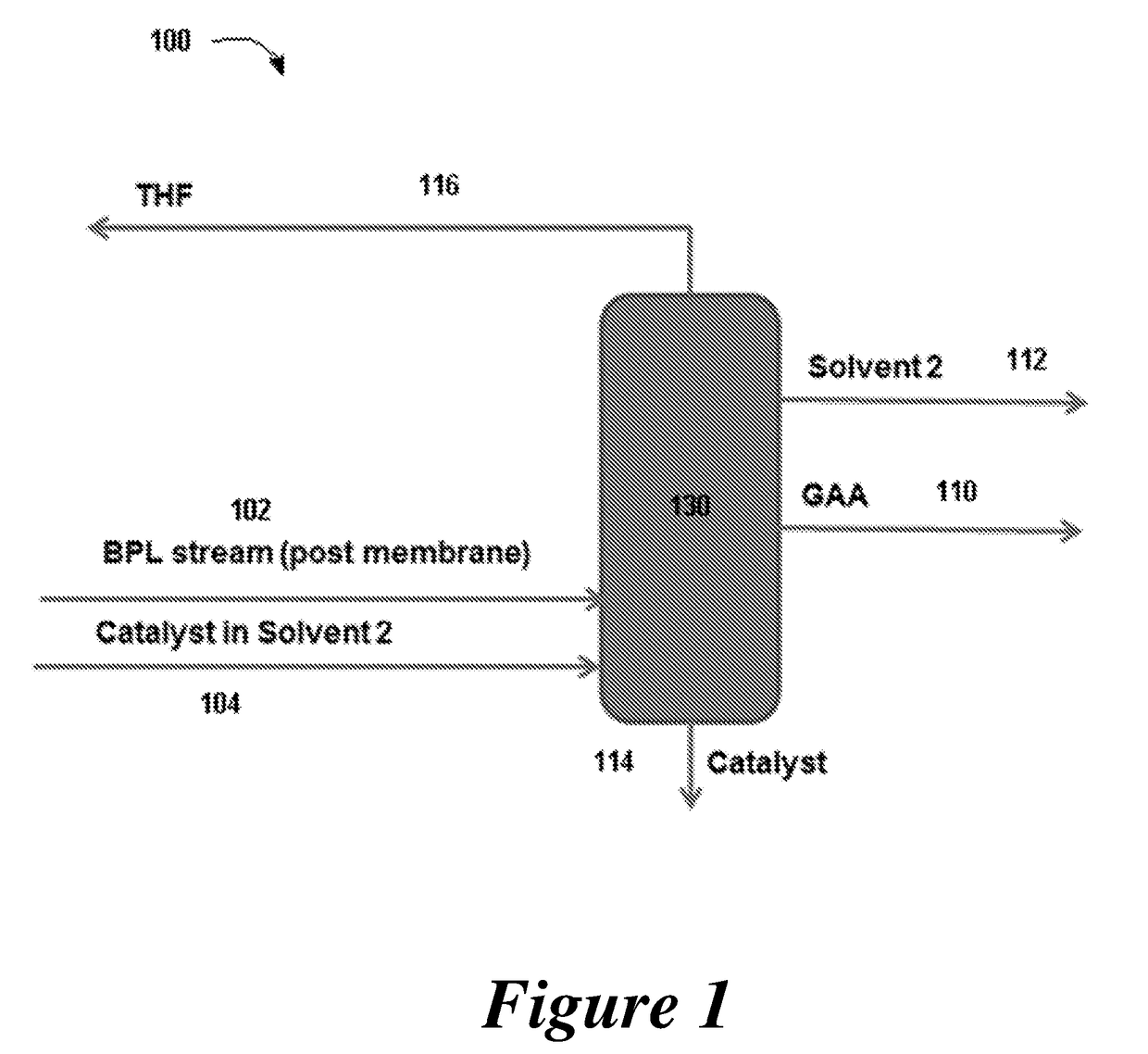
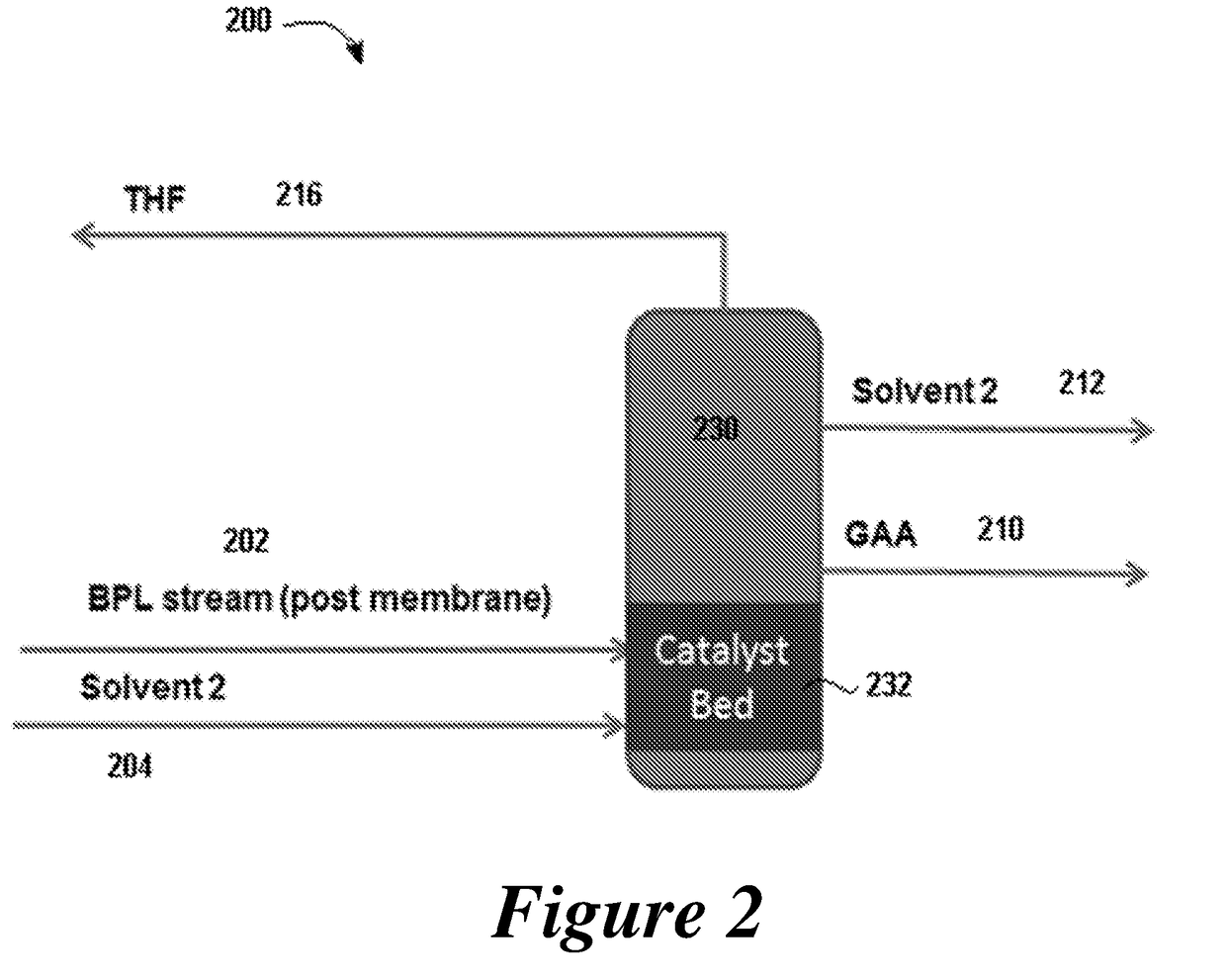
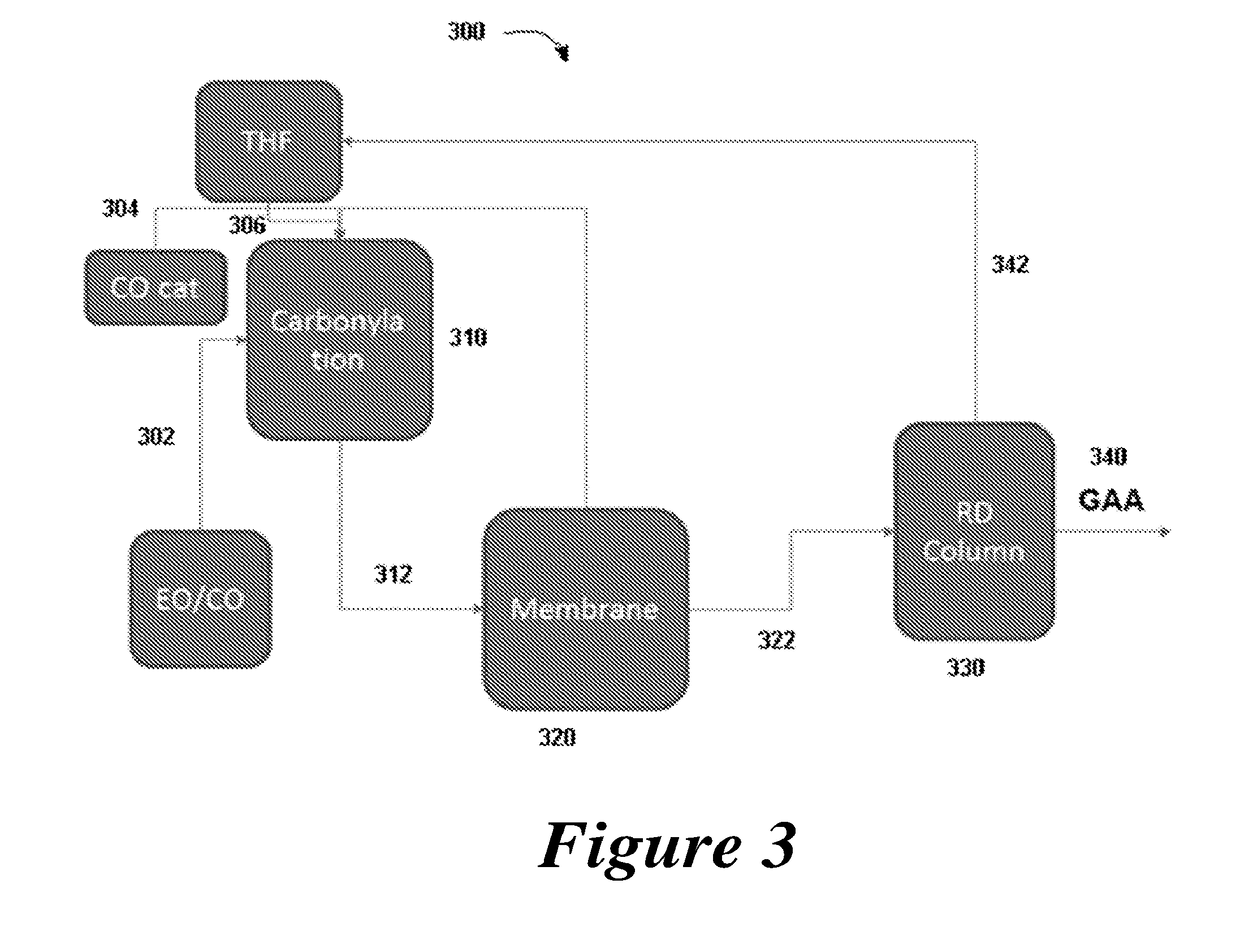
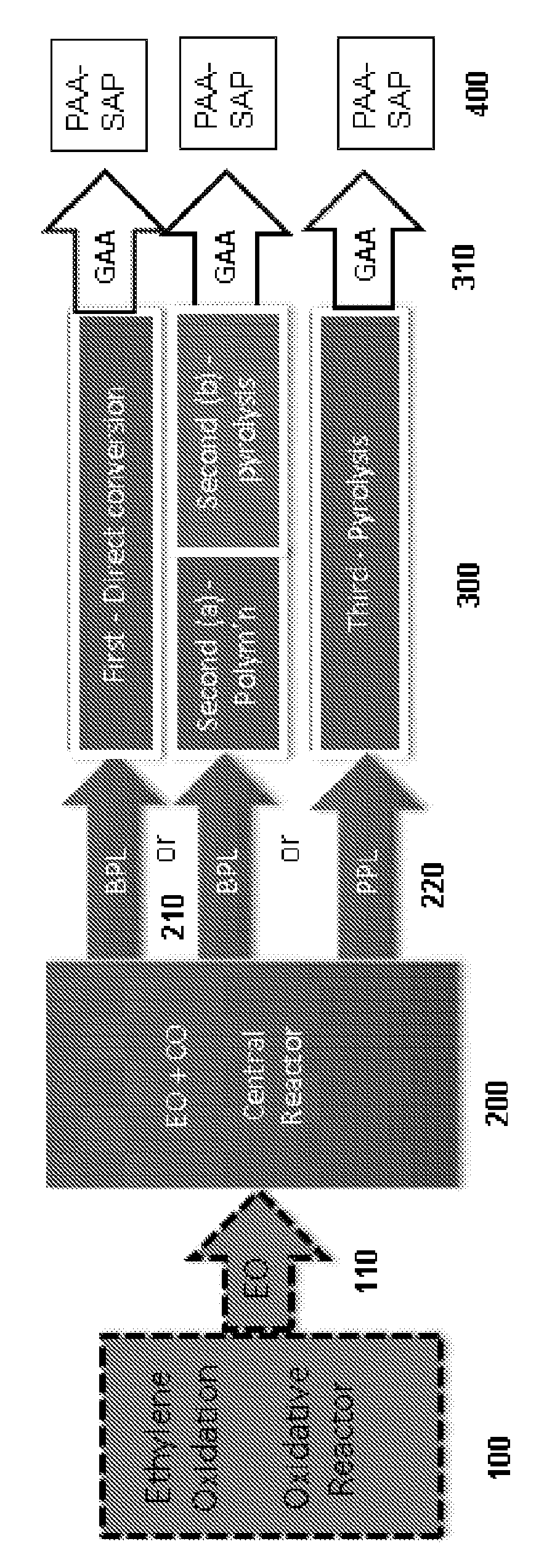
ActiveUS20180057619A1Efficient preparationHigh purityOrganic compound preparationPreparation from carboxylic acid esters/lactonesEthylene oxideSuperabsorbent polymer
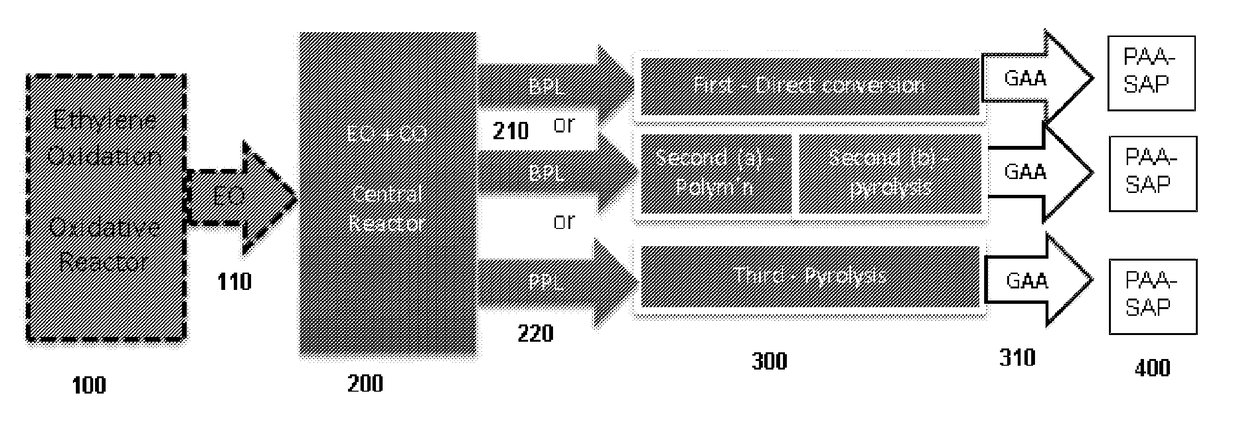
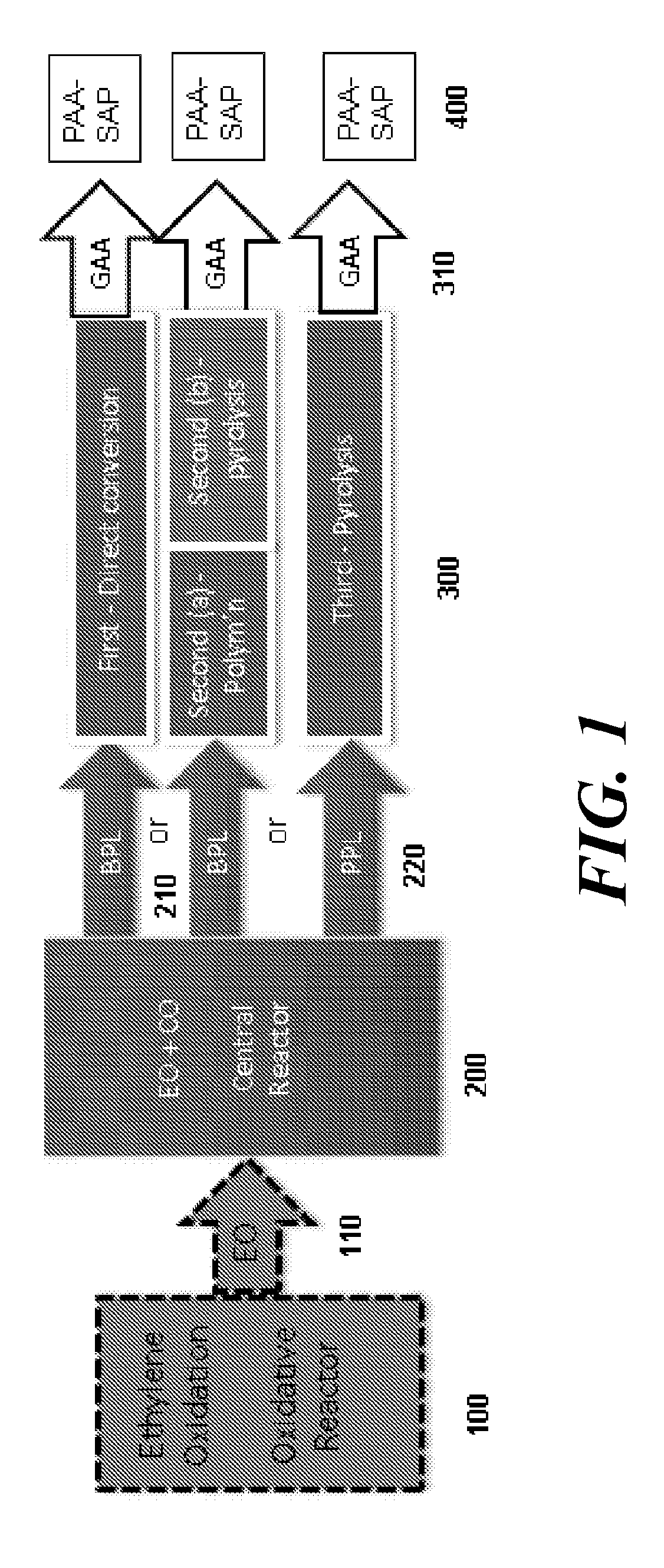
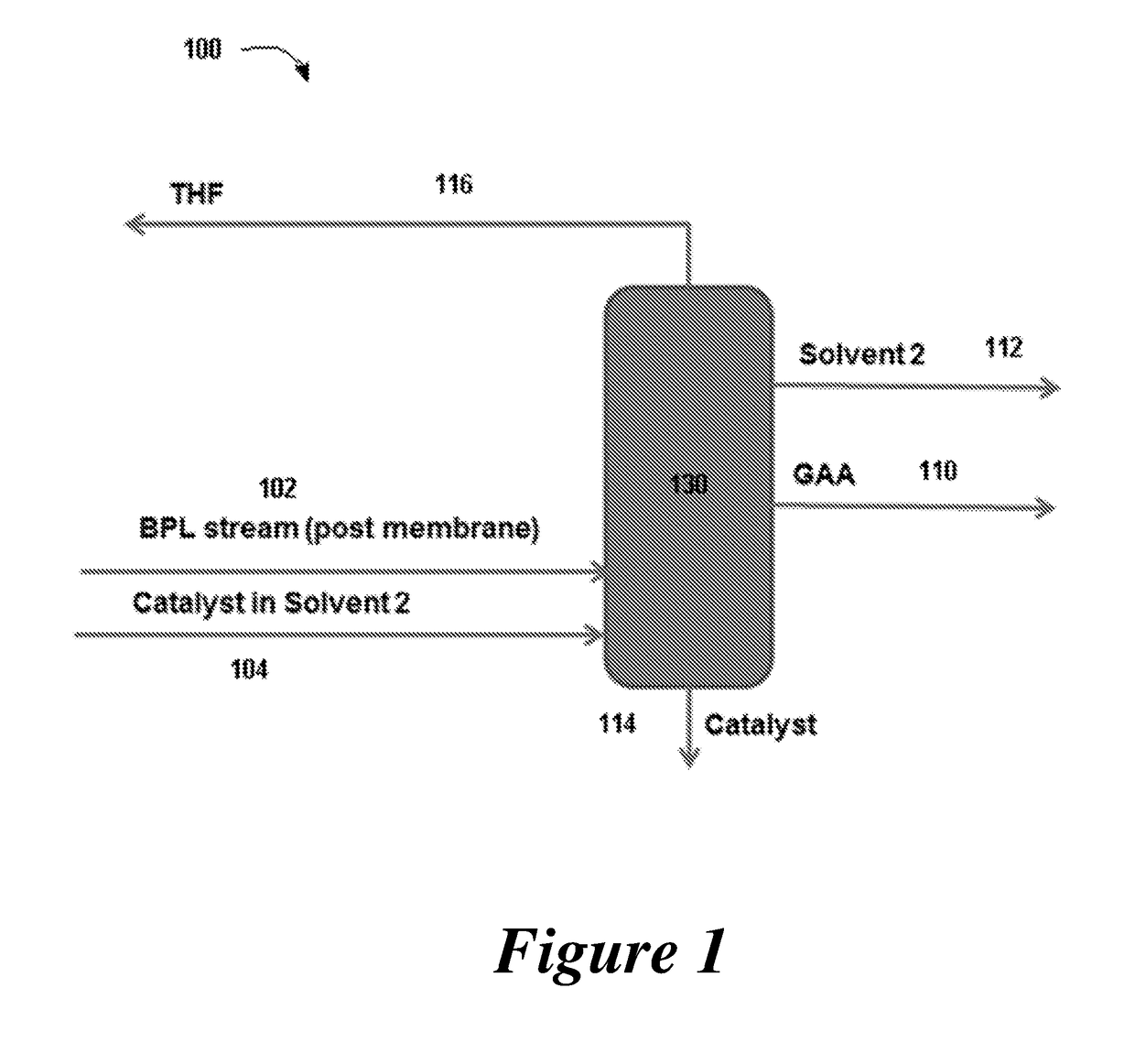
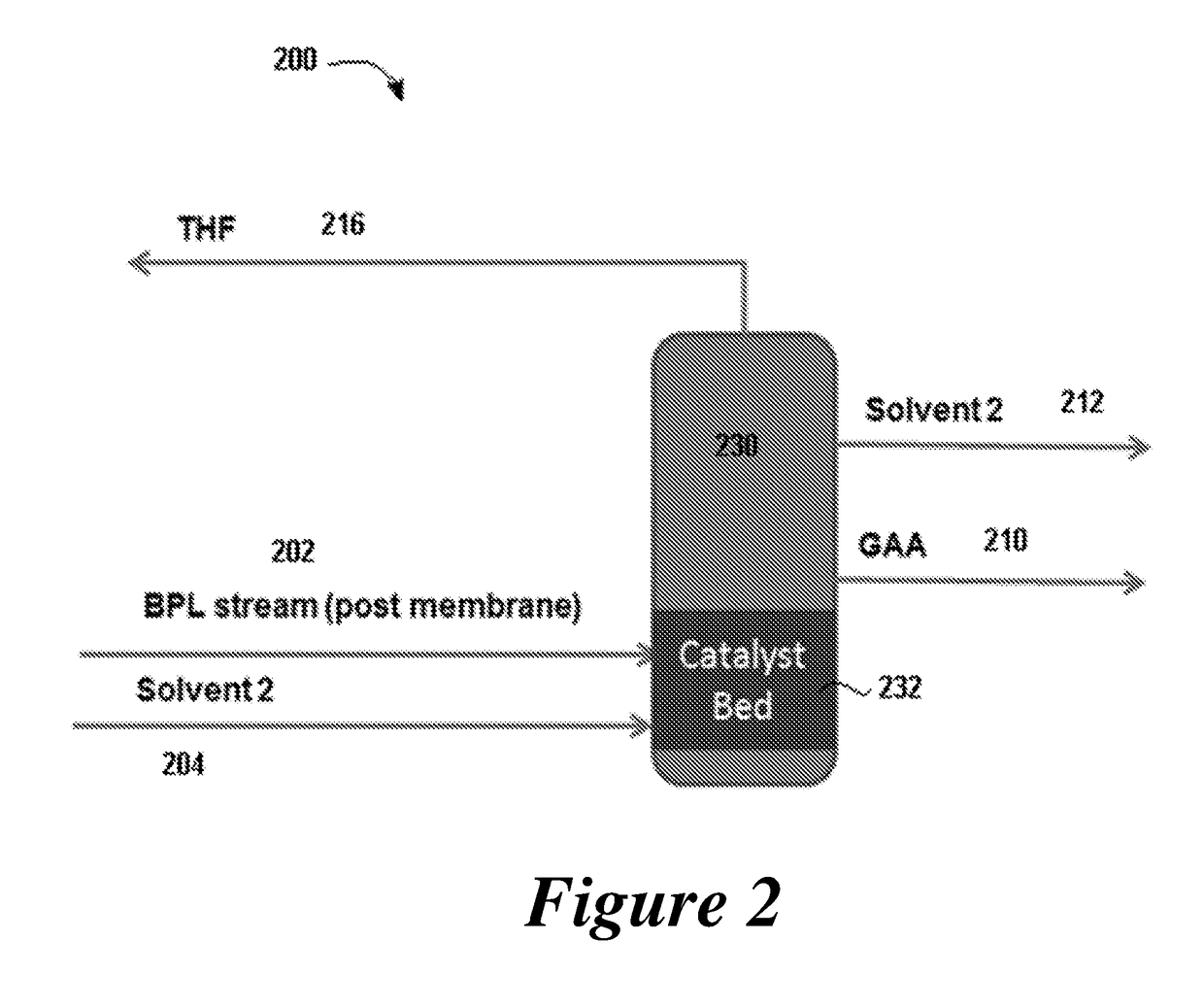
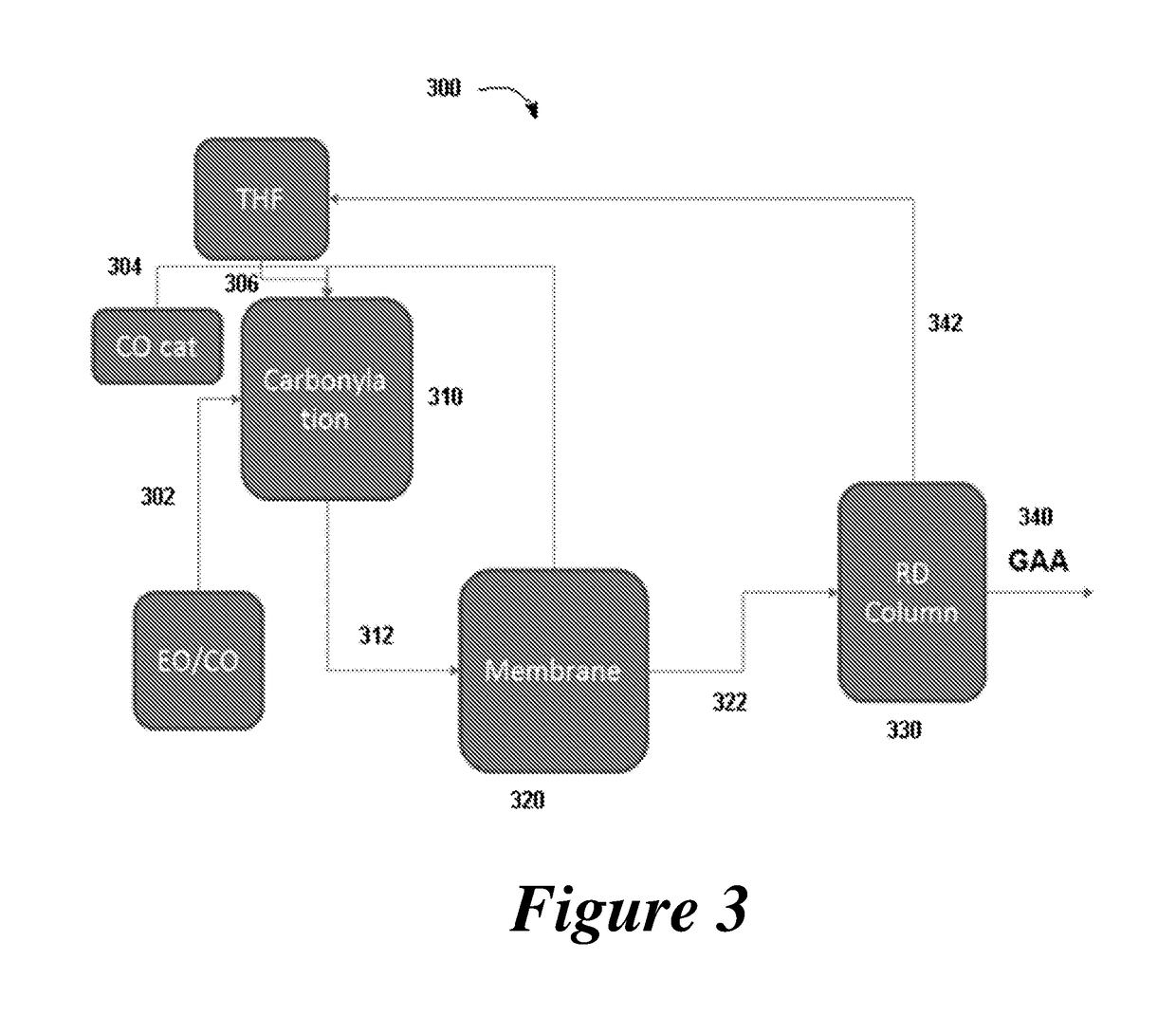
Disclosed are systems and methods for the production of polyacrylic acid and superabsorbent polymers from ethylene oxidation to form ethylene oxide. Reacting the ethylene oxide with carbon monoxide to form to beta propiolactone (BPL) or polypropiolactone (PPL), or a combination thereof. An outlet configured to provide a carbonylation stream comprising the BPL or PPL, or a combination thereof and using one or more reactors to convert BPL to acrylic acid or to convert at least some of the BPL to PPL, and then to convert PPL to acrylic acid. An outlet configured to provide a PPL stream to a second reactor tm to convert at least some of the PPL to AA or a third reactor to convert at least some of the PPL to AA. The outlet configured to provide an AA stream to a fourth reactor to convert the AA to polyacrylic acid.
Owner:NOVOMER INC
Distillation process for production of acrylic acid
Provided are integrated processes for the conversion of beta propiolactone to acrylic acid. Systems for the production of acrylic acid are also provided.
Owner:NOVOMER INC
Process for production of acrylic acid
ActiveUS10099988B2Promote aggregationReduce adverse effectsOrganic chemistry methodsChemical industryBiochemistryBeta-Propiolactone
Provided are integrated processes for the conversion of beta propiolactone to acrylic acid. Systems for the production of acrylic acid are also provided.
Owner:NOVOMER INC
Distillation process for production of acrylic acid
ActiveUS20180354882A1Energy inputPreparation from carboxylic acid esters/lactonesDistillation methodLactone
Provided are integrated processes for the conversion of beta propiolactone to acrylic acid. Systems for the production of acrylic acid are also provided.
Owner:NOVOMER INC
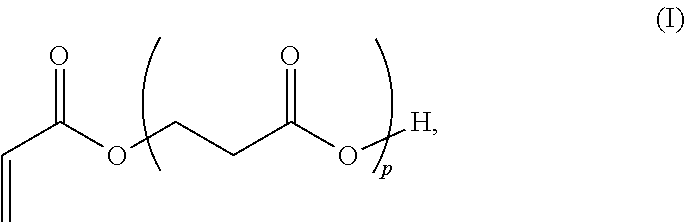
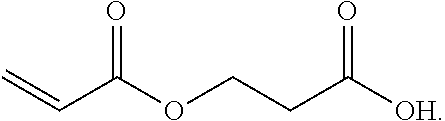
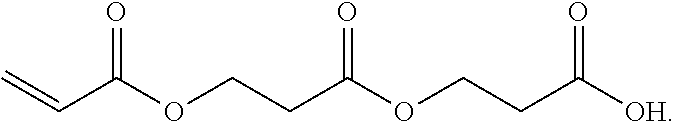
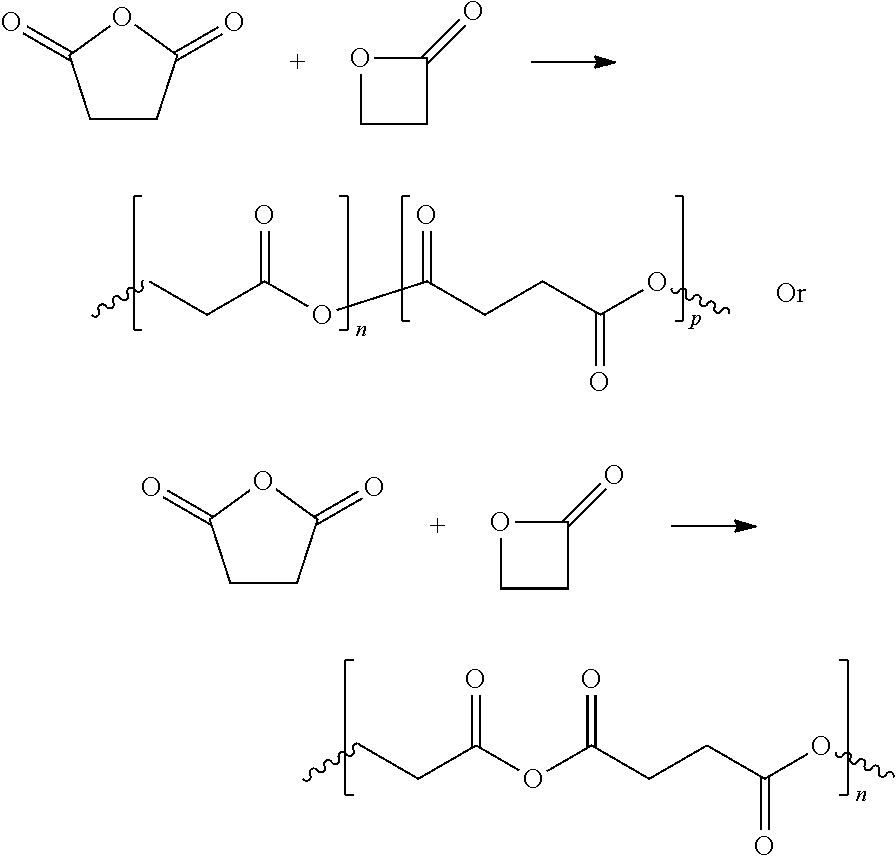
Beta-propiolactone based copolymers containing biogenic carbon, methods for their production and uses thereof
Provided herein are methods and systems for producing biodegradable beta-propoiolacone-based polyester polymers from renewable EO and CO on an industrial scale.
Owner:NOVOMER INC
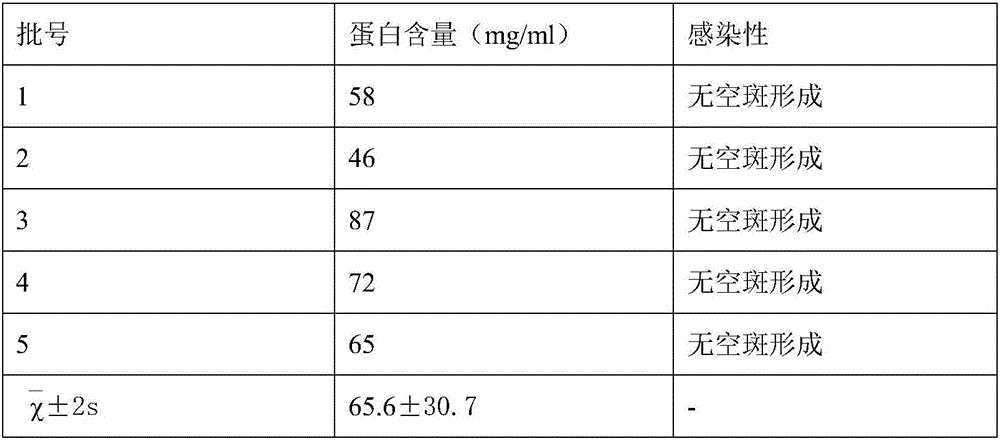
Method for preparing purified foot-and-mouth disease vaccine
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
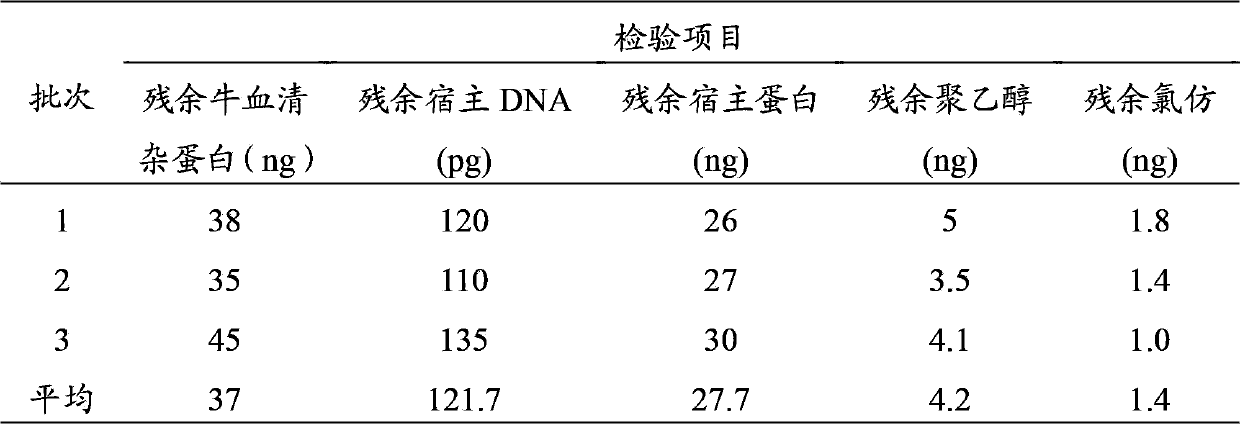
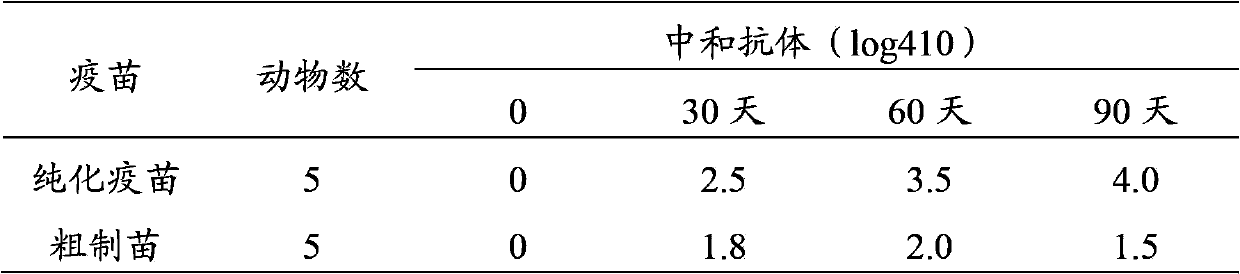
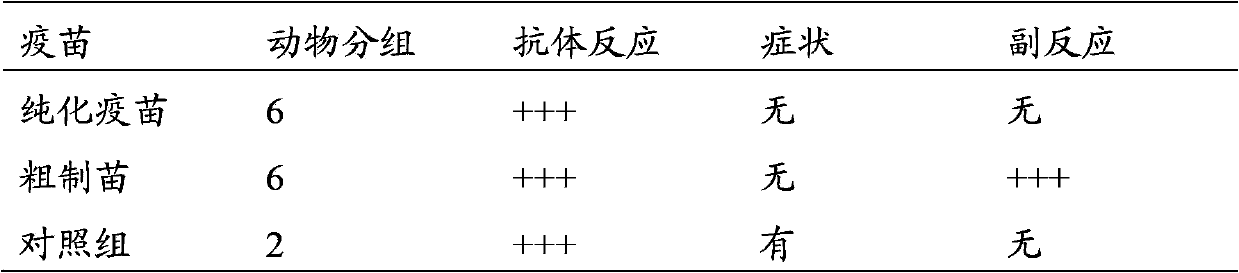
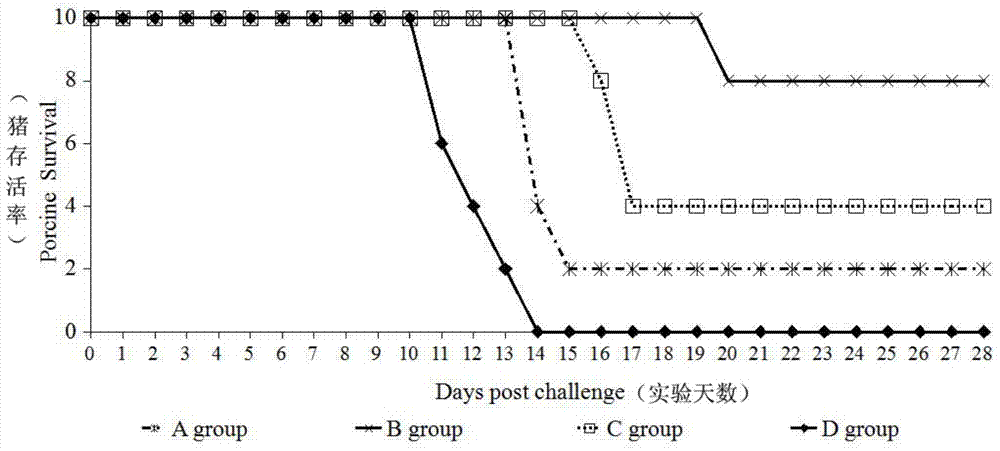
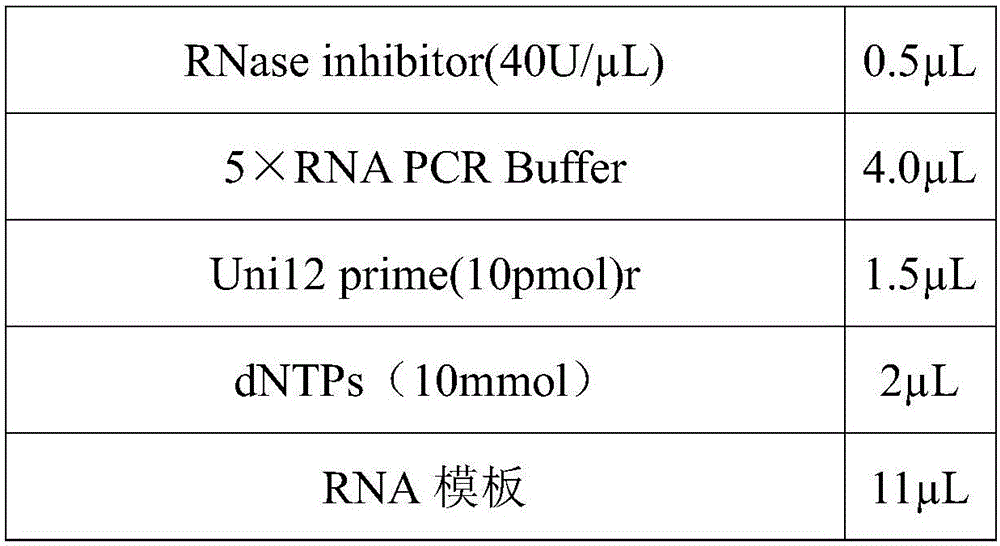
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
Preparation method and application of swine vaccine specific swine spleen transfer factor (TF)
ActiveCN103566370AReduce the use volumeEasy to mix and prepareAntiviralsAntibody ingredientsHigh concentrationDead volume
The invention discloses a preparation method of a swine vaccine specific swine spleen transfer factor (TF). The preparation method comprises the following steps: slaughtering a swine with a positive swine vaccine antibody to harvest the swine spleen; preserving the swine spleen at low temperature; unfreezing the swine spleen; removing fasciae; mincing the swine spleen; homogenizing the pulp; filling the homogenized pulp into a bottle and storing the pulp; repeatedly freezing and thawing the pulp; centrifuging the pulp; carrying out microfiltration; carrying out ultrafiltration; carrying out inactivation; regulating the pH value; regulating the osmotic pressure; removing bacteria; and detecting the quality. The preparation method has the advantages that the pH value of water for injection is regulated to 4-6 in the pulp homogenizing step, thus being beneficial to increasing of the yields of ribose and polypeptide; a box type membrane coating is adopted for tangential flow filtration, so that the dead volumes of system residues are small and linear amplification production is easy to achieve; a 1-3KD box type membrane coating is adopted to carry out tangential flow nanofiltration on a TF crude product, thus preparing the high-concentration TF and improving the using effects; phenol red is used as an acid-base indicator, thus being convenient for clients to observe the pH value of the TF; the TF uses beta-propiolactone for inactivation instead of traditional formaldehyde and an osmotic pressure regulation process is added, thus being beneficial to combined immunization of the TF and vaccines.
Owner:派生特(福州)生物科技有限公司 +2
Preparation method of inactivated vaccine for iridovirus of grouper
ActiveCN102178945AImproving immunogenicityHigh activityViral antigen ingredientsAntiviralsFreeze and thawEmbryo
The invention relates to a preparation method of an inactivated vaccine for the iridovirus of a grouper, comprising the following steps of: inoculating the iridovirus to embryonic cells of the grouper in a logarithmic phase by taking an embryonic fine system of the grouper as an amplification system of the iridovirus; repeatedly freezing and thawing and centrifugalizing after complete lesion, and inactivating an obtained iridovirus solution at 4 DEG C for 16 hours by using beta-propiolactone with the final concentration of 1:500 so as to obtain the inactivated iridovirus vaccine. The inactivated iridovirus vaccine is applied to a juvenile immunized Malabar grouper, and an iridovirus counteracting result shows that relative protection ratio is more than 90 percent after 15 days. The preparation method has the advantages of easiness and convenience for operation, simple equipment requirement and good repeatability and keeps good immunogenicity of the iridovirus under the precondition of efficiently inactivating the iridovirus, thereby having good immune protection effect; in addition, the invention can be used for the preventive immune of the grouper, thereby enhancing the survival rate and the culturing efficiency of cultured groupers.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Pharmaceutical Compositions Comprising A Pancreatic Enzyme Preparation With Viral Infectivity Reduced Below A Significant Level And Methods Of Preparing And Using The Same
InactiveUS20140017223A1Reduce alkylation activitySure easySsRNA viruses positive-sensePeptide/protein ingredientsAmylasePorcine circovirus
The present invention provides for pharmaceutical compositions comprising pancreatic enzyme preparations (PEPs) with viral infectivity reduced below significant levels and having high enzymatic activity. The PEPs can comprise lipases, proteases, amylases, non-enveloped viruses (e.g., porcine parvovirus (PPV), porcine circovirus type 2 (PCV-2), porcine encephalomyocarditis virus (EMCV)), and enveloped viruses (e.g., vesicular stomatitis virus (VSV), and influenza A (IFA)). The present invention also includes methods of treating pancreatic insufficiency by administering these pharmaceutical compositions and methods of making the same by treating the PEP with beta-propiolactone (BPL) to reduce viral infectivity.
Owner:APTALIS PHARMA CANADA
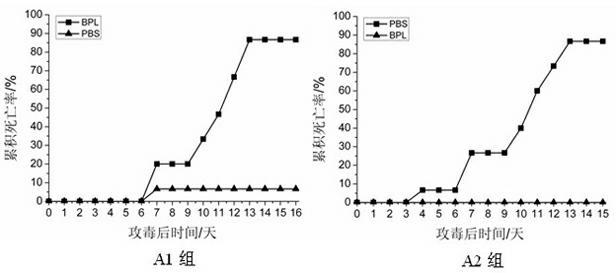
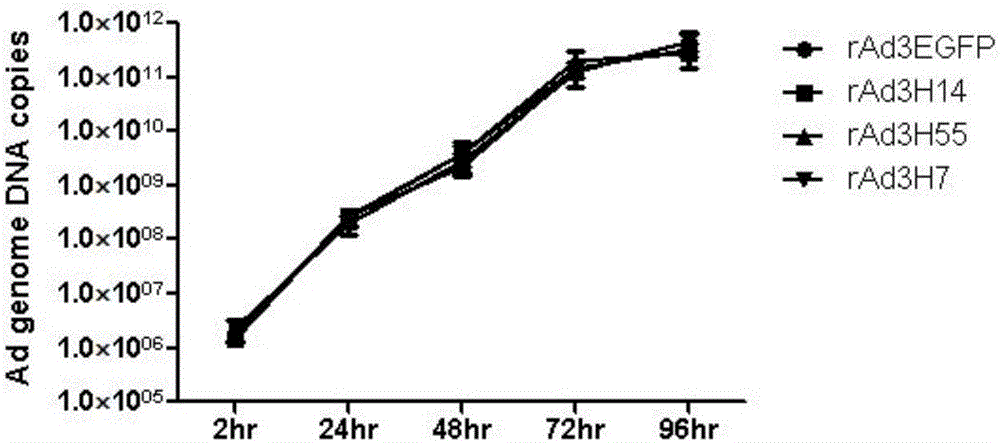
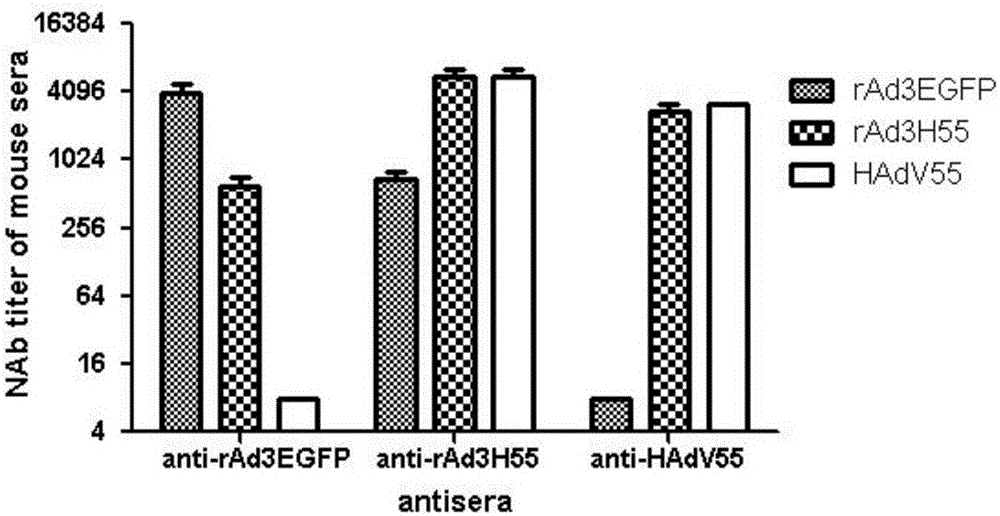
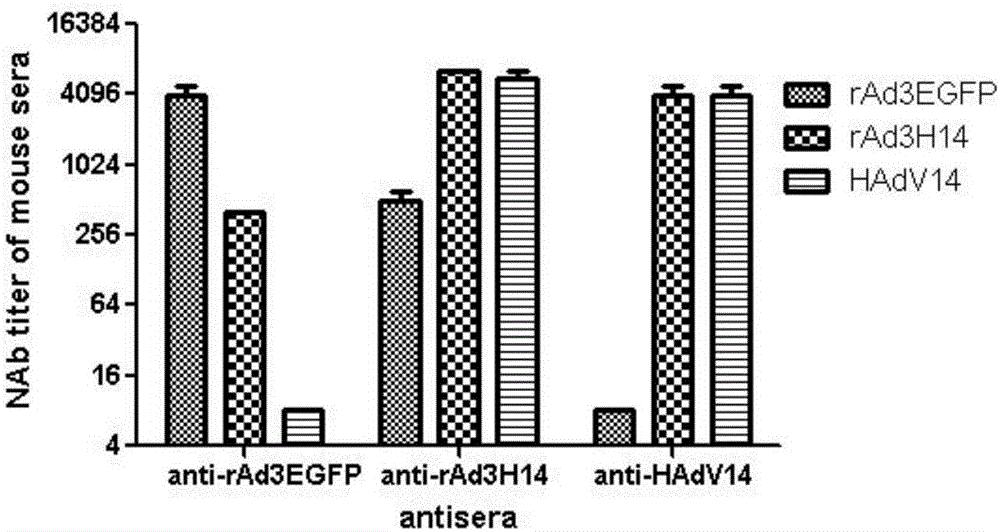
Recombinant adenovirus and tetravalent adenovirus vaccine and preparation method thereof
ActiveCN106318916ANo recombinationHigh neutralization potencyViral antigen ingredientsVirus peptidesHuman typeSerotype
The invention discloses a recombinant adenovirus and tetravalent adenovirus vaccine and a preparation method thereof. The tetravalent recombinant adenovirus vaccine contains a recombinant type 3 adenovirus strain, a recombinant type 7 adenovirus strain, a recombinant type 14 adenovirus strain and a recombinant type 55 adenovirus strain. The preparation method disclosed by the invention comprises the following steps: preparing recombinant shuttle plasmids containing hexon gene segments, and performing in-bacteria homologous recombination with a recombinant human type 3 adenovirus strain, thereby obtaining a recombinant adenoviral genome in which the hexon gene segments are replaced by type 7, type 14 and type 55; transfecting cells, rescuing to obtain recombinant human type 3, 7, 14 and 55 recombinant adenoviruses with different main capsid protein-hexon proteins; purifying, mixing according to the same protein content, and inactivating by using beta-propiolactone, thereby obtaining the tetravalent adenovirus vaccine. The tetravalent adenovirus vaccine is capable of inducing neutralizing antibody responses to four types of serotype adenoviruses, and the neutralizing titer is 500-1000.
Owner:GUANGZHOU GIR MEDICINE CO LTD +1
Systems and processes for polyacrylic acid production
ActiveUS10428165B2Efficient preparationHigh purityOrganic compound preparationPreparation from carboxylic acid esters/lactonesEthylene oxideSuperabsorbent polymer
Disclosed are systems and methods for the production of polyacrylic acid and superabsorbent polymers from ethylene oxidation to form ethylene oxide. Reacting the ethylene oxide with carbon monoxide to form to beta propiolactone (BPL) or polypropiolactone (PPL), or a combination thereof. An outlet configured to provide a carbonylation stream comprising the BPL or PPL, or a combination thereof and using one or more reactors to convert BPL to acrylic acid or to convert at least some of the BPL to PPL, and then to convert PPL to acrylic acid. An outlet configured to provide a PPL stream to a second reactor tm to convert at least some of the PPL to AA or a third reactor to convert at least some of the PPL to AA. The outlet configured to provide an AA stream to a fourth reactor to convert the AA to polyacrylic acid.
Owner:NOVOMER INC
Anti-stripping cyanoacrylate adhesive and preparation method thereof
ActiveCN105315933AImprove impact resistanceImprove anti-stripping performanceNon-macromolecular adhesive additivesProtein adhesivesEthyl cyanoacrylateGelatin
The invention discloses an anti-stripping cyanoacrylate adhesive. The anti-stripping cyanoacrylate adhesive is prepared from, by weight, 30-50 parts of alpha-ethyl cyanoacrylate, 0.5-4 parts of EVA, 2-5 parts of beta-propiolactone, 0.5-1 part of modified titanium oxide, 8-12 parts of gelatin, 5-10 parts of polyacrylate, 2-8 parts of galactomannan, 3-8 parts of hydroxyethyl cellulose and 1-1.5 parts of coupling agent. The invention further discloses a preparation method of the anti-stripping cyanoacrylate adhesive. The anti-stripping cyanoacrylate adhesive is high in curing speed, good in water resistance, high in impact resistance, large in bonding strength, good in anti-stripping performance, small in use amount, free of toxicity and beneficial to environmental protection.
Owner:ZHEJIANG TIME NEW MATERIAL
Pigeon paramyxovirus 1 strain AF-1 and application thereof
InactiveCN105936893AImproving immunogenicityHigh proliferative titerSsRNA viruses negative-senseViral antigen ingredientsAluminum StearateInfection induced
The invention discloses a pigeon paramyxovirus 1 strain AF-1 and an application thereof. The strain is a pigeon paramyxovirus 1 strain AF-1, and the preservation number of the strain is CCTCC NO:V 201613. A method for preparing an inactivated vaccine through using the pigeon paramyxovirus 1 strain AF-1 comprises the following steps: culturing the pigeon paramyxovirus 1 strain AF-1 to obtain an AF-1 strain virus solution, and adding beta-propiolactone to the AF-1 strain virus solution; inactivating the obtained virus solution to obtain a mixed solution; adding tween-80 to obtain an aqueous solution; adding aluminum stearate to white oil while stirring until the obtained oil is completely transparent, and adding tween-80 to obtain an oil solution; and slowly adding the aqueous solution to the oil solution, placing the obtained solution mixture in an emulsifying tank, and emulsifying the solution mixture to obtain the inactivated vaccine. The inactivated vaccine helps pigeons to effectively resist infection induced by pigeon paramyxovirus 1, greatly reduces the loss, promotes the development of the pigeon industry, is safe and effective, and can be applied to production practices.
Owner:武汉智健动物保健有限公司
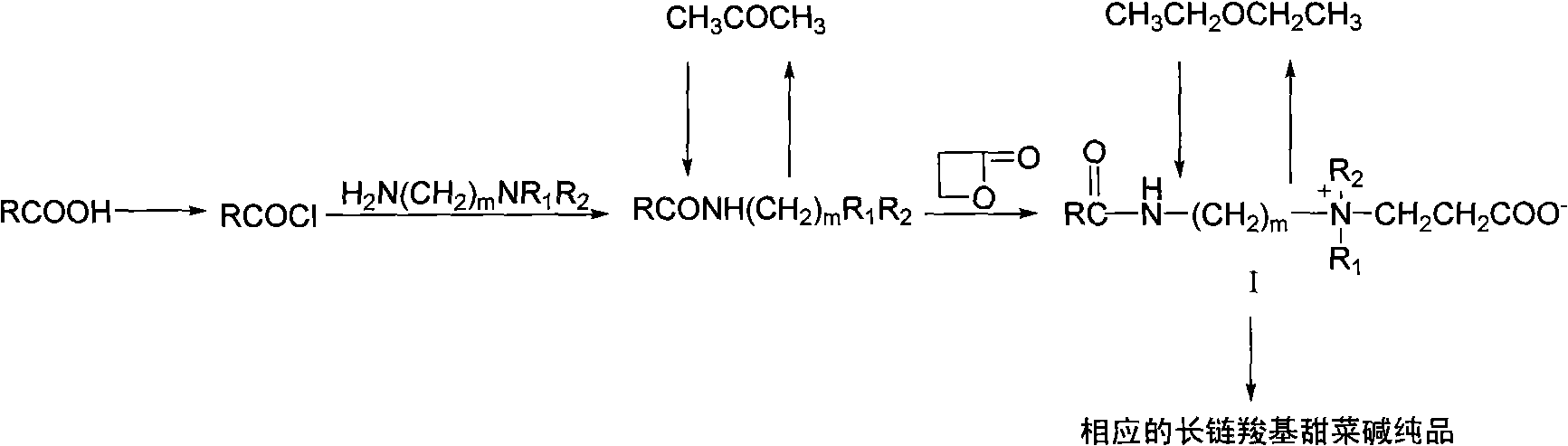
Long-chain carboxyl betaine surfactant and preparation method thereof
InactiveCN101618302AHigh purityTransportation and packagingMixingLong chain fatty acidInorganic salts
The invention relates to a long-chain carboxyl betaine surfactant and a preparation method thereof, and belongs to the technical field of surfactants. The preparation method is characterized in that: long-chain fatty acid is subjected to halogenation, amidation and beta-propiolactone quaternization reactions in turn to obtain corresponding long-chain carboxyl betaine. The method has the advantages that simple separation and purification means can obtain pure surfactants without inorganic salt and organic salt, and provides possibility for the application of the surfactant in a wider range.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Varicella virus inactivated vaccine used for people and preparation method thereof
ActiveCN107174658AComplete inactivation effectShort time to inactivate pathogensViral antigen ingredientsAntiviralsVirus inactivationFreeze-drying
The invention relates to the field of biotechnology, and particularly provides a varicella virus inactivated vaccine used for people and a preparation method thereof. The preparation method comprises the following steps: mixing beta-propiolactone and varicella virus concentrated solution at a volume ratio of (1:2000) to (1:8000), carrying out inactivation for 12-36h at a temperature of 3-5 DEG C; then, at the temperature of 34-37 DEG C, carrying out hydrolysis for 1.5-3h to obtain inactivated virus liquid; carrying out purification, split charging and freeze-drying on the inactivated virus liquid to obtain the inactivated vaccine. The varicella virus inactivated vaccine prepared with the method is safe and effective, is slight in vaccination reaction and is more suitable for crowds who can not use attenuated live vaccines and have poor immunologic functions.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
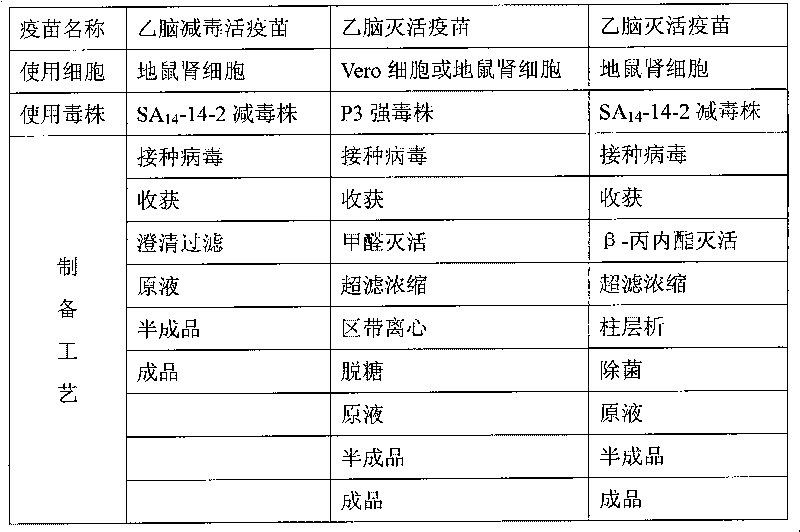
Preparation method of vaccine for epidemic encephalitis B
InactiveCN101732708AImprove securityAvoid damageViral antigen ingredientsInactivation/attenuationSodium bicarbonateViral culture
The invention relates to a preparation method of a vaccine for epidemic encephalitis B, which comprises the processes of cell culture, virus propagation and vaccine preparation. After cells overgrow with a single layer, a seed culture of viruses and a culture solution are inoculated, and the use quantity of the seed culture of the viruses is that every 100cm<2> of cells is inoculated with 1ml of seed culture of the viruses. The culture solution of the viruses mainly comprises 90-95% of MEM culture solution, 3-5% of calf serum and 7.5% of sodium bicarbonate solution the pH of which is regulated to 7.2-7.6. The vaccine is prepared by adding human serum albumin with the final concentration of 0.3% after being clarified and filtered. The invention adopts an SA14-14-2 attenuated strain to produce an encephalitis purified vaccine. Compared with a vaccine produced by a P3 strain, the attenuated strain is safer and more effective. The invention has the advantages that: (1) the attenuated strain is used for preparing the purified vaccine, which has good safety; (2) the inactivation effect of inactivating the viruses by using beta-propiolactone is good, and an inactivator is naturally degraded and has no residue; and (3) the column chromatography purification property is mild, and the injury to the viruses is small.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
CA-193 virus strain and application thereof to preparation of inactivated vaccine
ActiveCN106367398ARich preparation methodImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsFiltrationVaccine virus
The invention discloses a CA-193 virus strain (Coxackievirus A16) and application thereof to preparation of an inactivated vaccine. For the application, MRC-5 cells are used for proliferating the CA-193 virus strain; virus proliferation liquid is subjected to centrifugation, concentration and column chromatography filtration and purification after inactivation by formaldehyde or beta-propiolactone; and a CA16 inactivated vaccine is obtained. The invention provides the vaccine virus strain capable of being used for preparing a human CA16 inactivated vaccine; the subtype of the vaccine virus strain belongs to the main epidemiological subtype B1 in Chinese Mainland; the vaccine virus strain has good growth characteristics on the MCR-5 cells; and the virus titer (7.5 to 8.251gTCID50 / ml) with high stability can be obtained. A preparation method of the vaccine is complete; the impurity content is low; the immunogenicity and the immunizing protection performance are good; meanwhile, cell culture substrates are MRC-5 cells; the safety is high; and the international and CFDA specifications and requirements on the vaccine research and development can be well met.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Preparation method and application of swine vaccine specific cattle spleen transfer factor (TF)
ActiveCN103565834AReduce the use volumeEasy to mix and prepareUnknown materialsImmunological disordersHigh concentrationDead volume
The invention discloses a preparation method of a swine vaccine specific cattle spleen transfer factor (TF). The preparation method comprises the following steps: slaughtering a cattle with a positive swine vaccine antibody to harvest the cattle spleen; preserving the cattle spleen at low temperature; unfreezing the cattle spleen; removing fasciae; mincing the cattle spleen; homogenizing the pulp; filling the homogenized pulp into a bottle and storing the pulp; repeatedly freezing and thawing the pulp; centrifuging the pulp; carrying out microfiltration; carrying out ultrafiltration; carrying out inactivation; regulating the pH value; regulating the osmotic pressure; removing bacteria; and detecting the quality. The preparation method has the advantages that the pH value of water for injection is regulated to 4-6 in the pulp homogenizing step, thus being beneficial to increasing of the yields of ribose and polypeptide; a box type membrane coating is adopted for tangential flow filtration, so that the dead volumes of system residues are small and linear amplification production is easy to achieve; a 1-3KD box type membrane coating is adopted to carry out tangential flow nanofiltration on a TF crude product, thus preparing the high-concentration TF and improving the using effects; phenol red is used as an acid-base indicator, thus being convenient for clients to observe the pH value of the TF; the TF uses beta-propiolactone for inactivation instead of traditional formaldehyde and an osmotic pressure regulation process is added, thus being beneficial to combined immunization of the TF and vaccines.
Owner:派生特(福州)生物科技有限公司 +1
Bursin extraction method
InactiveCN107129530AIncrease profitEasily brokenDepsipeptidesPeptide preparation methodsHydrolysateBursa fabricius
The invention discloses a bursin extraction method. The method includes: well mixing pretreated chicken bursa fabricius with water for injection, mashing and homogenizing; subjecting the homogenized bursa fabricius to twice extraction to obtain extract liquid, and adding beta-propiolactone for inactivation; hydrolyzing inactivated extract liquid to obtain hydrolysate; adopting a 0.22-0.45micron filter membrane for tangential flow microfiltration of the hydrolysate, collecting microfiltration permeation liquid, adopting a 1-5kD filter membrane for tangential flow ultrafiltration, and collecting ultrafiltration permeation liquid; adjusting an osmotic pressure of the ultrafiltration permeation liquid to obtain isoosmotic solution, adopting 0.1-0.22micron sterilization filter for sterilization, and collecting liquid namely bursin. The bursin extraction method is simple and convenient, the bursa fabricius utilization rate is increased, production cost is reduced, production time is shortened, and the extracted bursin can serve as an immunity enhancer, a feed additive, an auxiliary therapeutic agent and the like.
Owner:派生特(福州)生物科技有限公司
Anti-vibrio alginnolyficus nutrition enhancer for sea cucumbers
The invention discloses an anti-vibrio alginnolyficus nutrition enhancer for sea cucumbers. The total weight ratio of an inactivated product to a nutritional feed is 1:(2-10). The preparation process comprises the following steps: bacteria is multiplied and washed to collect bacterial cells; then the bacterial cells are allowed to stand for 2 hours at the temperature of 37 DEG C for hydrolyzing after beta-propiolactone inactivation processing, and is sprayed and dried into the nutritional feed for drying for 24-36 hours; the total weight ratio of the inactivated product to the nutritional feed is 1:(2-10); the product water content is 2-5%; end products are prepared. The anti-vibrio alginnolyficus nutrition enhancer prepared by using the method provided by the embodiment of the invention, is suitable for nursery ponds and culture ponds of the sea cucumbers; the treating process is simple; the time consumption is reduced; the preparation cost is lower than that of the traditional method; the powder has a long preservation time and is free of pollution. The anti-vibrio alginnolyficus nutrition enhancer is of important practical significance for control over vibrio alginnolyficus for large-scale culture of sea cucumbers.
Owner:DALIAN DETONG BIO TECH DEV
Absorbent polymers, and methods and systems of producing thereof and uses thereof
Provided herein are absorbent polymers produced from beta-propiolactone, and methods and systems of producing such polymers. The beta-propiolactone may be derived from ethylene oxide and carbon monoxide. The absorbent polymer may be bio-based and / or biodegradable. The absorbent polymers may be used for diapers, adult incontinence products, and feminine hygiene products, as well as for agricultural applications.
Owner:NOVOMER INC
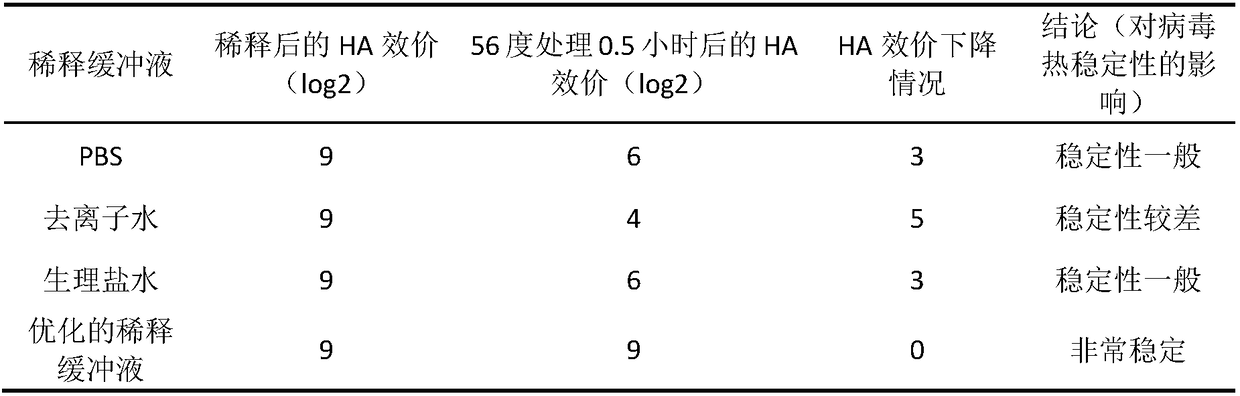
Inactivated vaccine prepared by Newcastle disease virus heat stable strain and preparation method thereof
ActiveCN108379575AImprove thermal stabilityOvercome stabilitySsRNA viruses negative-senseViral antigen ingredientsCold chainOil adjuvant
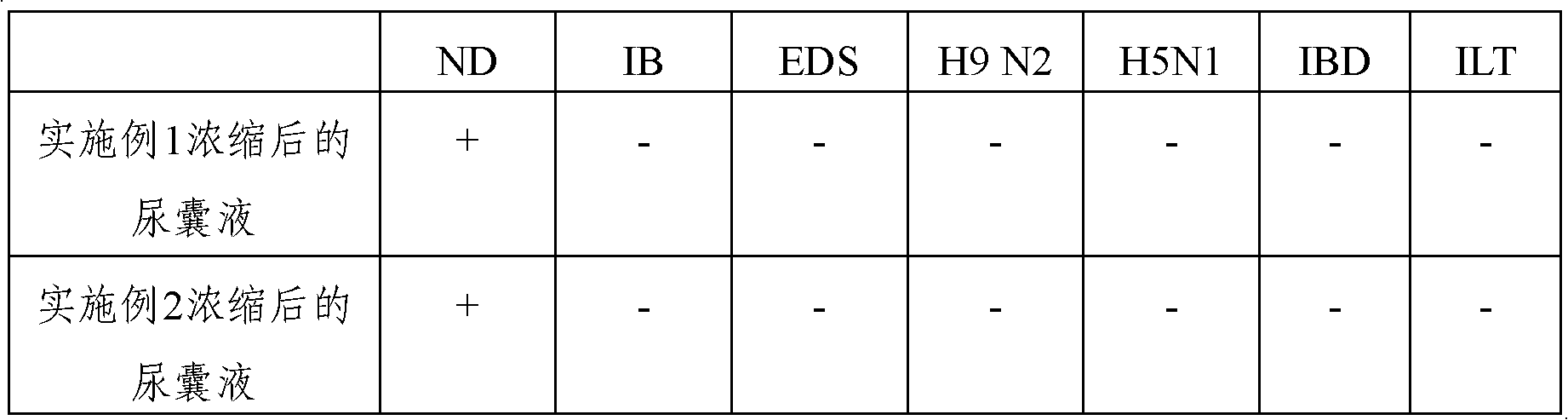
The invention discloses an inactivated vaccine prepared by a Newcastle disease virus heat stable strain and a preparation method thereof. The vaccine takes the Newcastle disease virus heat stable strain as a vaccine virus seed. The preparation method comprises the following steps: preparing viral allantoic fluid of the Newcastle disease virus heat stable strain, diluting the viral allantoic fluidwith optimized dilution buffer, and preparing and inspecting beta-propiolactone and oil-adjuvant inactivated vaccine. The heat stability of the vaccine is obviously higher than that of the other conventional inactivated vaccines, and the inactivated vaccine also has excellent immunogenicity, solves the problems of relatively poor stability, high dependency level on a cold chain system and the likein the conventional common Newcastle disease virus vaccine, and has wide application prospects.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
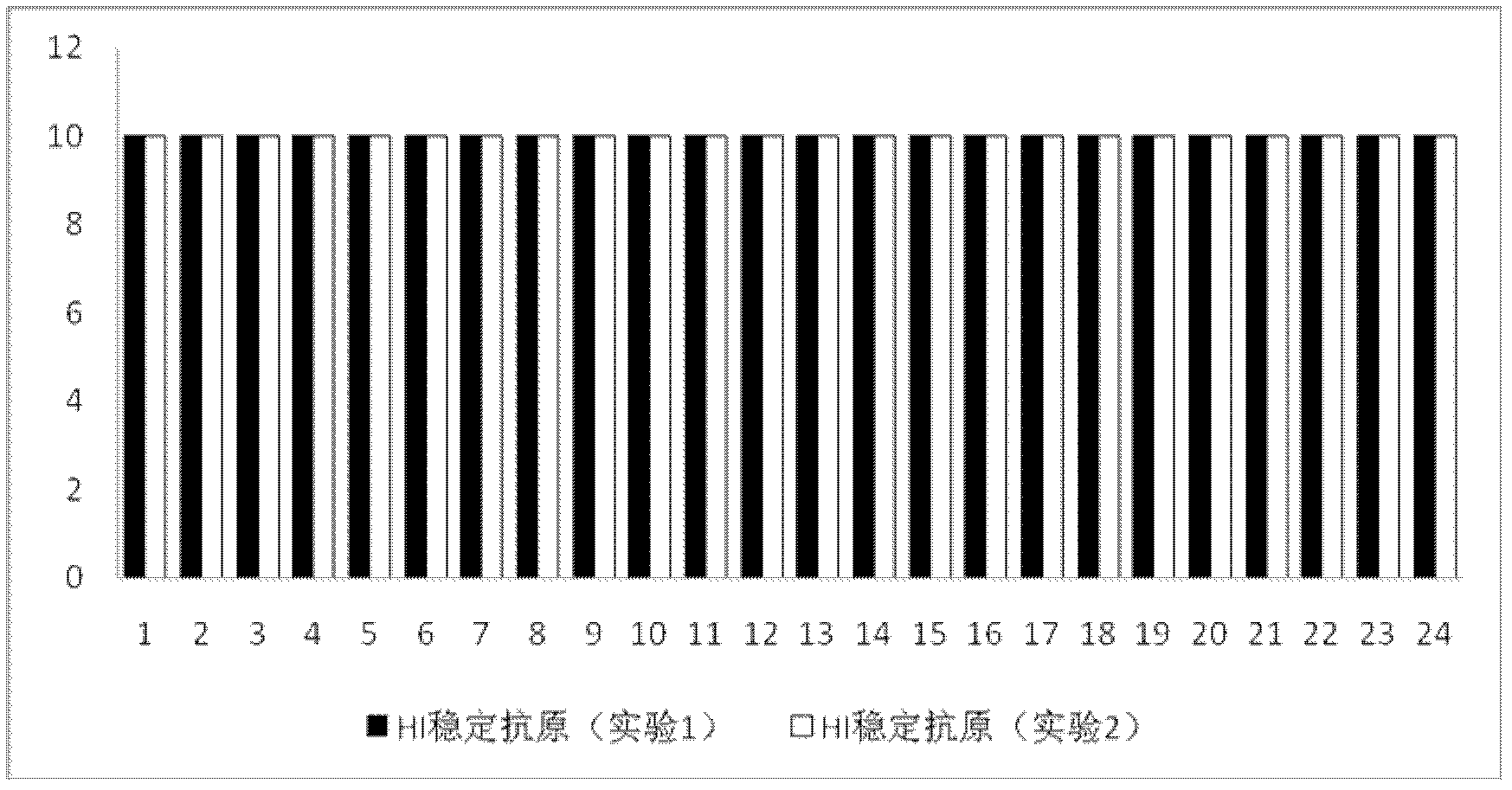
Preparation method of newcastle disease HI antigen
ActiveCN102352345AEasy to storeEasy to transportViral antigen ingredientsAntiviralsAntigenNewcastle disease virus NDV
The invention provides a preparation method of a newcastle disease HI antigen. The method comprises the following technical steps: (1) inoculating newcastle disease virus into a chick embryo, then harvesting allantoic fluid of the chick embryo and adding an inactivator, namely beta-propiolactone according to 0.25 per mill-0.5 per mill by volume of the allantoic fluid; (2) concentrating the inactivated allantoic fluid to 1 / 10-1 / 20 of the original volume and adding a stabilizer; (3) further using PBS (phosphate buffer saline) buffer solution in the volume which is 1 / 5-1 / 10 of that of the allantoic fluid before concentration for diluting the antigen; and (4) performing ultrasonic degradation, adding glycerol which accounts for 20%-30% of the final volume, oscillating and uniformly mixing so as to get the antigen which can be used. The prepared antigen has no risk of dispersing toxin during the use and the storage, and can reduce the damage to an environment and a human body due to the use of the live virus antigen, the product has good stability, the hematic coagulating rate of the product is unchanged by being preserved for above 24 months at the temperature of 2-8 DEG C, and the newcastle disease HI antigen can be used for detecting newcastle disease HI.
Owner:北京盛华四合生物科技有限公司
Inactivated vaccine for preventing monkey from SV40 virus infection and preparation method
The invention is concerned with a kind of inactivated vaccine and preparation method to anti-monkey SV40 virus, belonging to animal vaccine. It carries multiplication, inactivation and purification to prepare inactivated vaccine of anti-monkey SV40 virus. This vaccine is SV40 virus and adjuvant inactivated with beta-Propiolactone and the cubage of them is 2:1 to 3:2. Mix the said component with buffer solution and get Tween-20 with 0.03 to 0.05 mg / ml and glycine with 2.0 to 4.0mg / ml, and the virus content is 104.6 to 5.2TCID50 / ml. Purificate the SV40 virus liquid with 0 percent CPE to collect virus, inactivate and stop SV40 virus with beta-Propiolactone and use adjuvant absorb inactivated SV40virus. The specific antibody caused of small rat can identify SV40virus with security. The tested monkey can produce neutralizing antibody with singularity, and the neutralizing antibody with singularity can stop the infection of SV40virus to cell culturing matter during the test. This inactivated vaccine maybe has natural anti-infection function.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Preparation method and use of poultry vaccine-specific pig spleen transfer factor
ActiveCN103599534AReduce the use volumeEasy to mix and prepareUnknown materialsAntiinfectivesHigh concentrationFiltration
The present invention discloses a preparation method for a poultry vaccine-specific pig spleen transfer factor. The preparation method comprises: selecting poultry vaccine antibody-positive pigs, and slaughtering to obtain pig spleen; carrying out cold preservation; thawing the pig spleen; removing fascia; mincing; homogenizing; filling into a bottle, and storing; carrying out repeated freezing and thawing; carrying out centrifugation; carrying out microfiltration; carrying out ultra-filtration; inactivating; adjusting the pH value; adjusting an osmotic pressure; sterilizing; and testing quality. According to the present invention, in the homogenizing step, the pH value of water for injection is adjusted to 4-6 so as to easily increase yields of ribose and polypeptides; the box type member package tangential flow filtration is adopted, such that the residual dead volume of the system is small, and linear enlargement production is easily achieved; the transfer factor crude product is subjected to tangential flow nano-filtration through a 1-3 KD box type member package to prepare the high concentration transfer factor so as to increase a use effect; phenol red is adopted as a pH value indicator, such that customers easily observe the pH value of the transfer factor; and beta-propiolactone is adopted to replace the traditional formaldehyde to inactivate the transfer factor, and the osmotic pressure adjustment process is added so as to easily achieve transfer factor and vaccine combined immunization.
Owner:派生特(福州)生物科技有限公司 +1
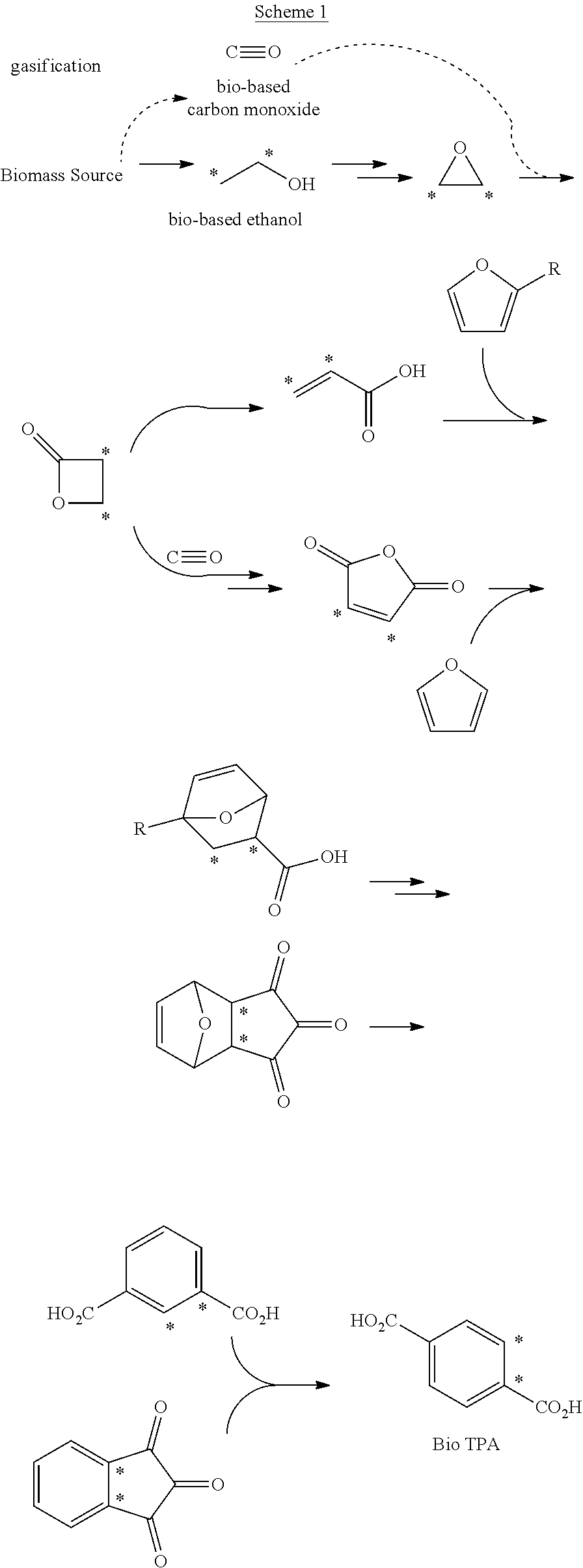
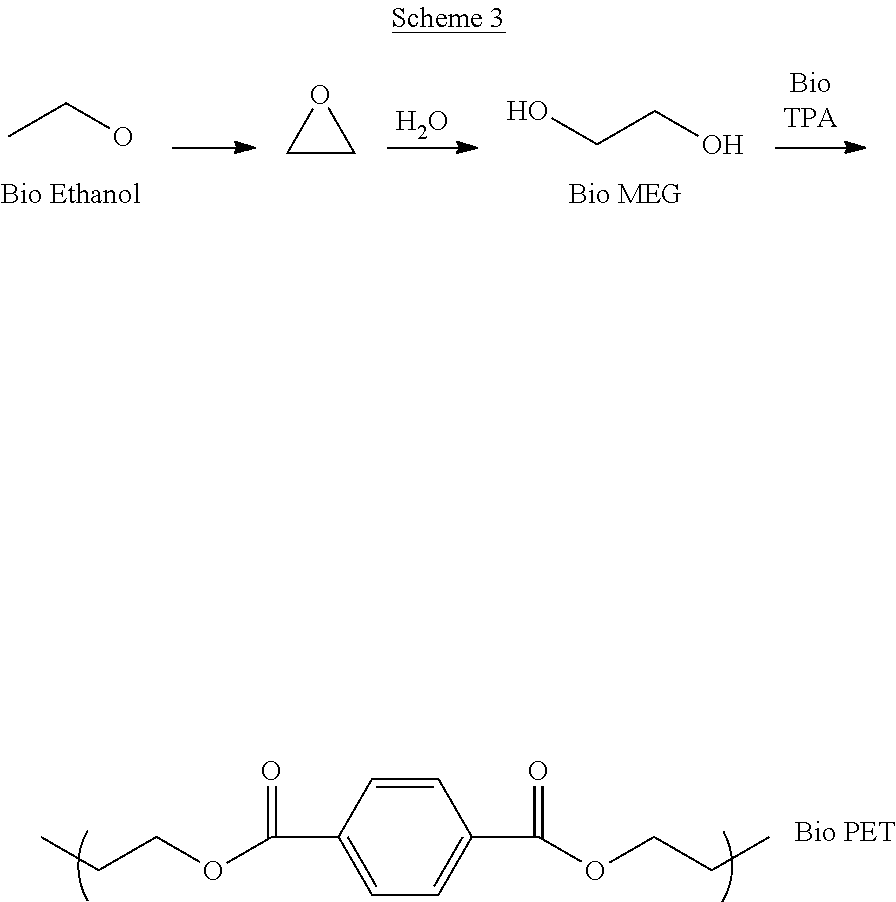
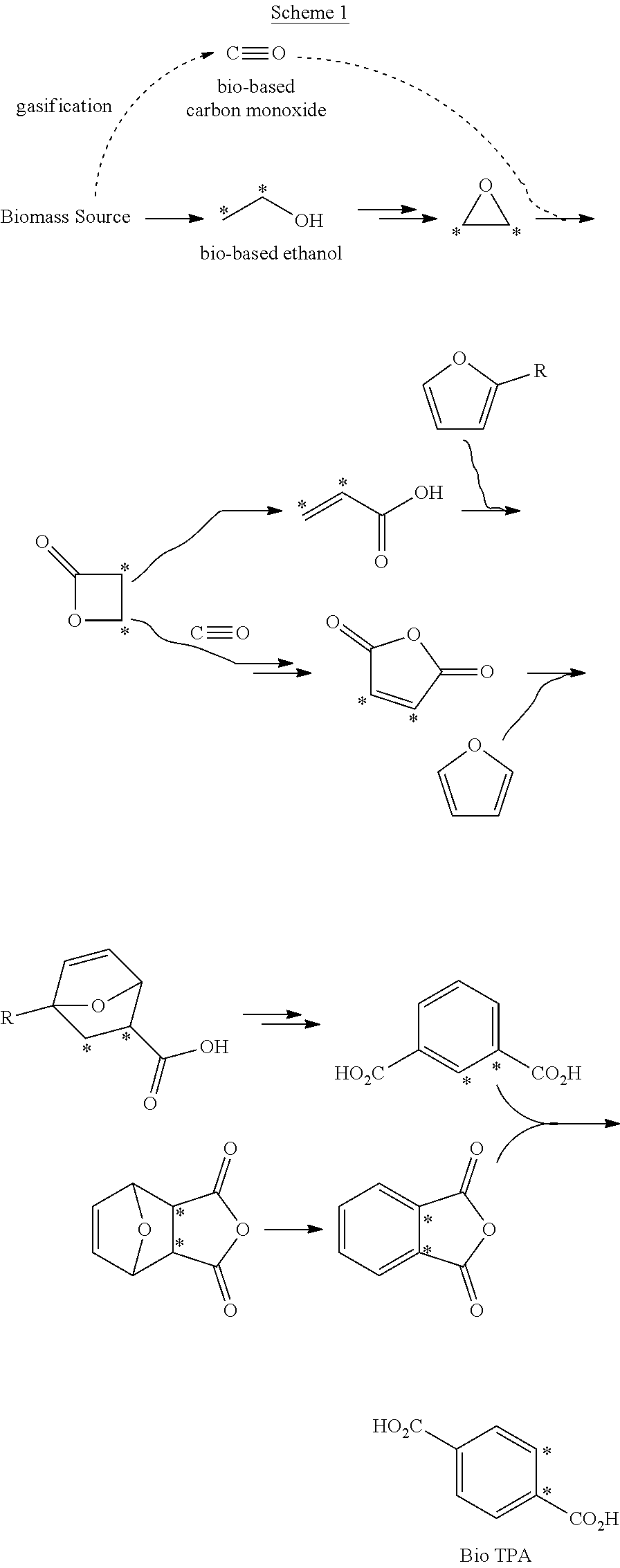
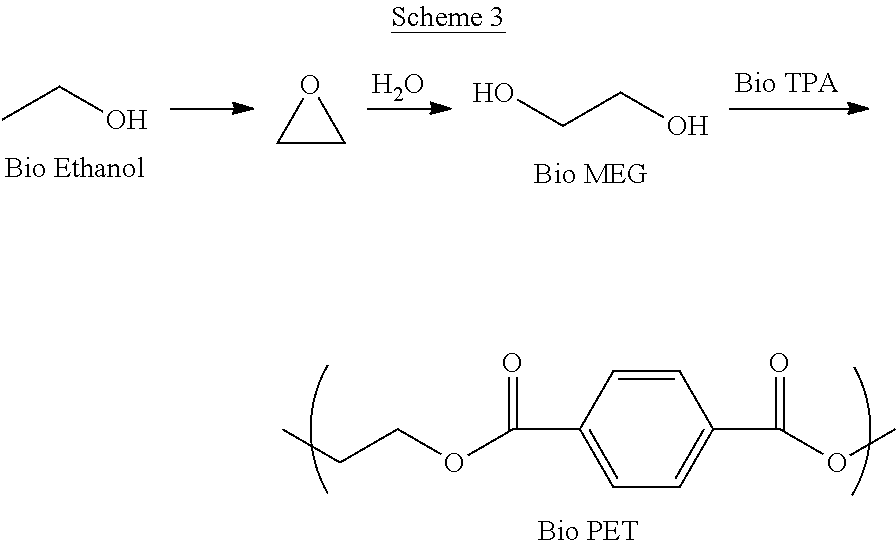
Processes for the production of polymer compositions from bio-based ethanol and compositions thereof
Owner:NOVOMER INC
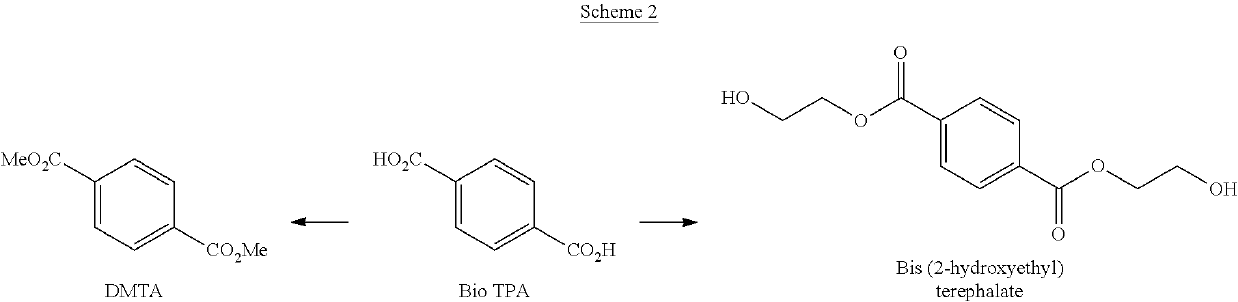
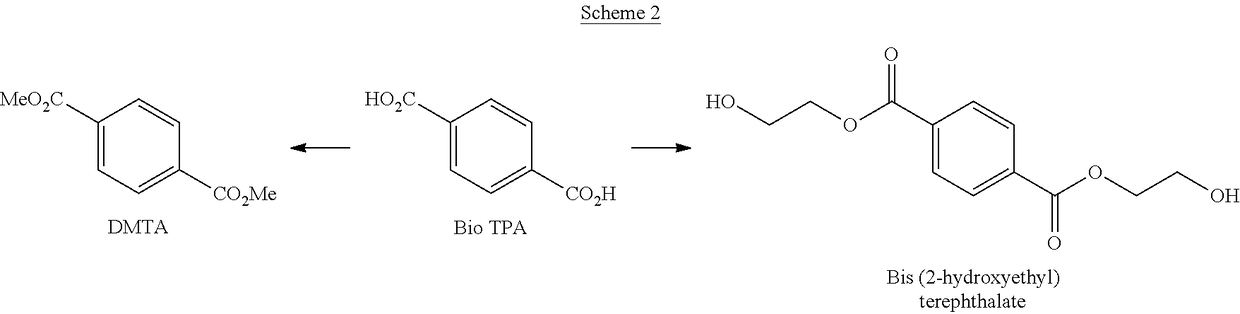
Processes for the production of terephthalate derivatives and compositions thereof
Process(es) produce compositions comprising dimethyl terephthalate (DMTA) for polymer chains having terephthalate moieties containing carbons from ethanol having at least two of the carbon atoms in the terephthalate ring that are fossil based. The compounds produced by the process(es) are DMTA polymers. The Henkel process converts ethanol to ethylene oxide, beta propiolactone, and / or terephthalic acid which can all serve as substituents producing DMTA by the process(es) disclosed. A production of DMTA by the process(es) disclosed herein result in DMTA with two ethanol-derived carbon atoms in the terephthalate ring with the carboxy group. In the production of terephthalate derived by process(es) disclosed herein by reaction of ethylene with dimethylfuran bond both of the ethylene-derived carbons to unsubstituted positions of the aromatic ring. DMTA polymer compositions produced by the process(es) disclosed herein are used as plastic molding compositions and as material for manufactured consumer goods packaging, most prominently in plastic water bottles.
Owner:NOVOMER INC
An environmentally friendly water scale inhibitor
InactiveCN106809961AEasy to operateHigh inhibitory effectScale removal and water softeningPolyethylene glycolPhytic acid
The invention relates to the field of water treatment agents and particularly relates to an environment-friendly water scale inhibitor. The environment-friendly water scale inhibitor is prepared from the following substances in parts by weight: 20-30 parts of phytic acid, 10-15 parts of ferrous oxide, 5-10 parts of glucose, 10-20 parts of maleic anhydride, 20-30 parts of sodium pyrosulfite, 25-32 parts of sodium gluconate, 15-27 parts of sodium tungstate, 30-40 parts of hydrogen peroxide, 15-30 parts of epoxysuccinic acid, 15-20 parts of calcium hydroxide, 10-15 parts of allyl polyethylene glycol monoether, 12-18 parts of beta-propiolactone, 5-12 parts of gamma-butyrolactone, 15-25 parts of vinyl-containing unsaturated double-bond carboxylic acid monomer and 45-55 parts of water. According to the environment-friendly water scale inhibitor provided by the invention, environment-friendly raw materials are adopted, the operation is simple, and the environment-friendly water scale inhibitor has a good scale inhibiting action; the used glucose and sodium gluconate are biodegradable, have an excellent scale inhibiting action and meanwhile also have a very good corrosion inhibiting action; and a polyether scale inhibitor structure contains a polyether group with high hydrophilicity and carboxylate radical ions capable of being chelated with calcium ions, so that the environment-friendly water scale inhibitor plays a role in efficiently inhibiting calcium phosphate and calcium carbonate scales of an industrial circulating cooling water system.
Owner:青岛城轨交通装备科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com