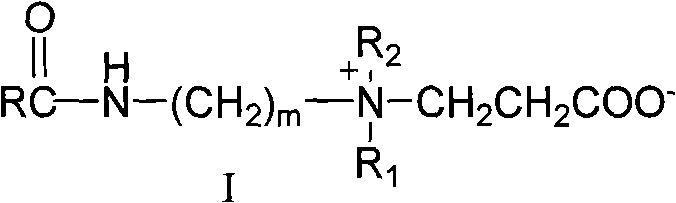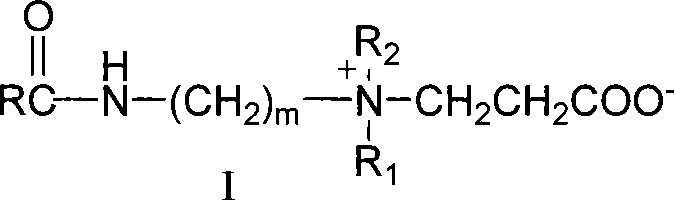Long-chain carboxyl betaine surfactant and preparation method thereof
A technology of surfactant and chain carboxyl, which is applied in the field of long-chain carboxy betaine surfactant and its preparation, which can solve the problems of environmental pollution, large solvent consumption, and difficulty in recycling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A 18 B 3 Synthesis of -2C
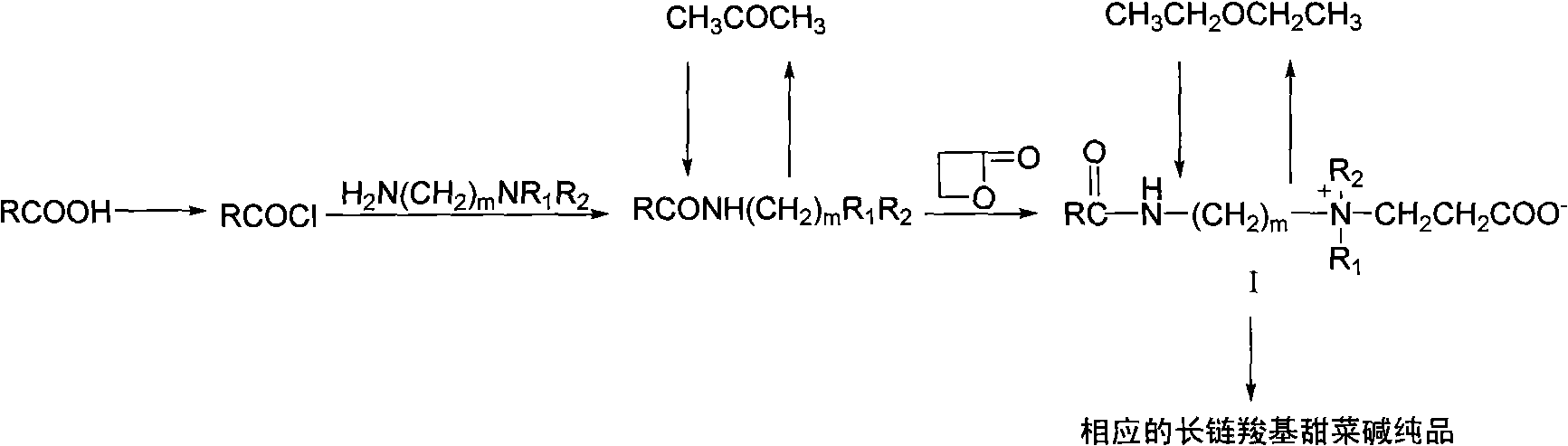
[0017] (1) Preparation of stearyl chloride: Take 0.1mol (28.5g) of stearic acid and place it in a reaction flask, heat it in an oil bath to 90°C, add 0.2mol (23.8g) of SOCl dropwise 2 , reflux, halogenation reaction 5h, stop the reaction, distill off excess SOCl 2 , to obtain a brown liquid that is stearyl chloride.
[0018] (2) Preparation of N,N-dimethyl-N'-stearamide: 0.105mol (10.7g) N,N-dimethylpropylenediamine was placed in a reaction flask, 200ml of acetonitrile was added, and 0.1 mol (30.4g) of stearyl chloride, cooled in an ice-water bath for 5h to obtain the crude product of a light yellow solid, and the crude product was washed with acetone to obtain N,N-dimethyl-N'-stearamide (A 18 B 3 ).
[0019] (3) Preparation of long-chain alkyl amido carboxy betaine: take 0.1mol (36.9g) of the above-mentioned tertiary amine intermediate A 18 B 3 And 0.12mol (8.6g) β-propiolactone was added to the reaction flask, 200ml ethyl acetate wa...
Embodiment 2
[0021] A 22U B 3 Synthesis of -2C
[0022] (1) Preparation of erucoyl chloride: Take 0.1mol (33.8g) of erucic acid (cis-n-docos-13-enoic acid) in a reaction flask, heat the oil bath to 40°C, add 100ml of CH 2 Cl 2 , add dropwise 0.2mol (23.8g) of SOCl 2 , reflux, halogenation reaction 5h, stop the reaction, distill off excess SOCl 2 , the brown liquid obtained is erucoyl chloride.
[0023] (2) Preparation of N,N-dimethyl-N'-erucamide: 0.105mol (10.7g) N,N-dimethylpropylenediamine was placed in a reaction flask, 200ml of acetonitrile was added, and 0.1mol was added dropwise (35.7g) erucoyl chloride, cooled and reacted in an ice-water bath for 5h, obtained a slightly yellow solid crude product, and the crude product was washed with acetone to obtain N,N-dimethyl-N'-long-chain alkylamide (A 22U B 3 ).
[0024] (3) Preparation of long-chain alkyl amido carboxy betaine: take 0.1mol (36.9g) of the above-mentioned tertiary amine intermediate A 22U B 3 and 0.12mol (8.6g) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com