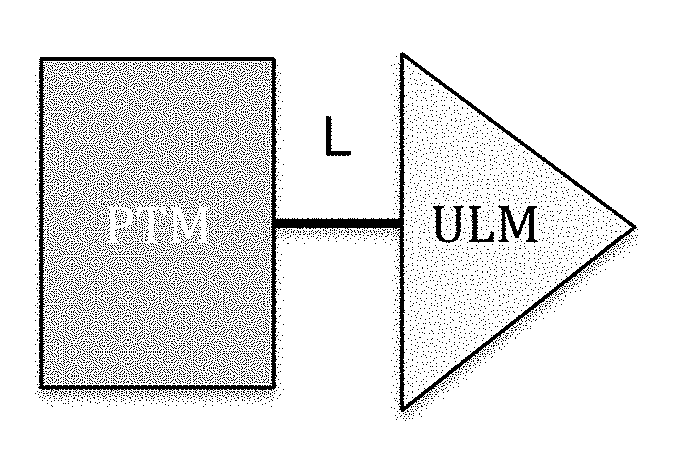
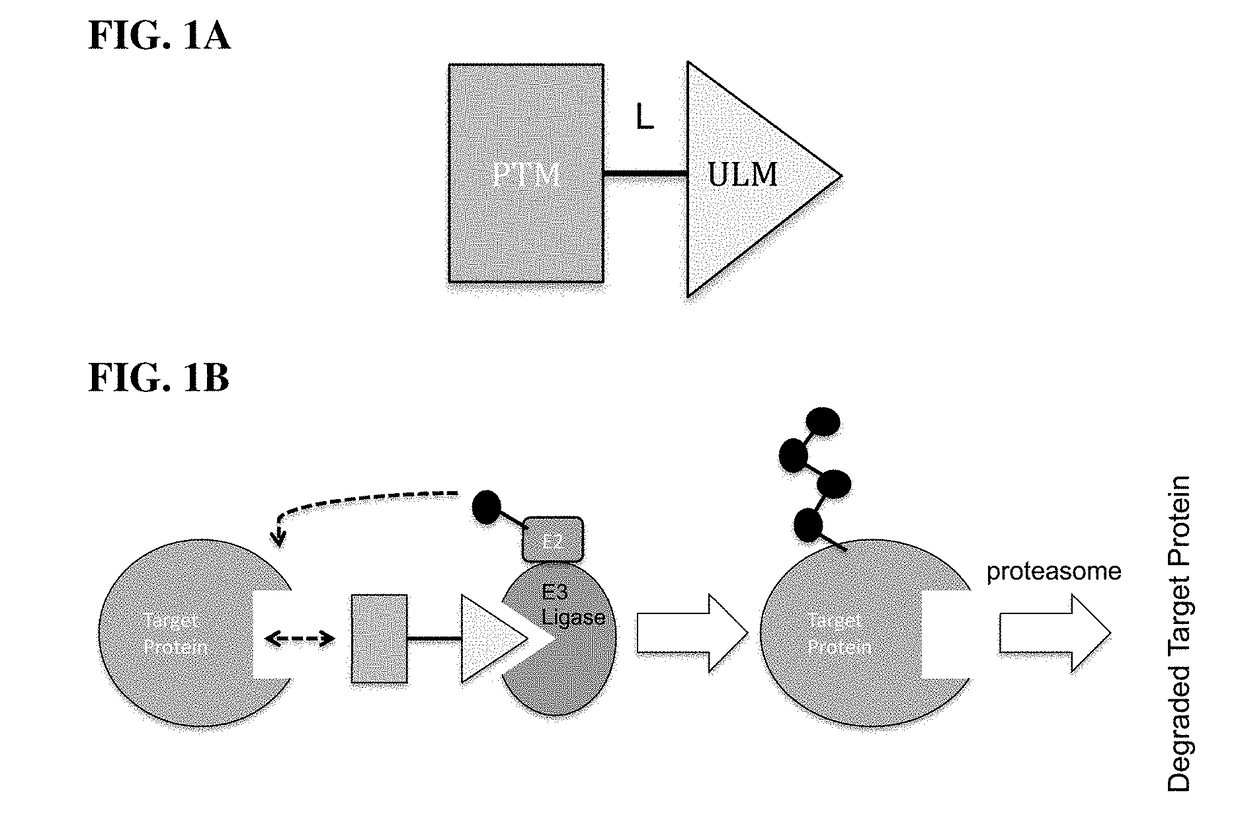
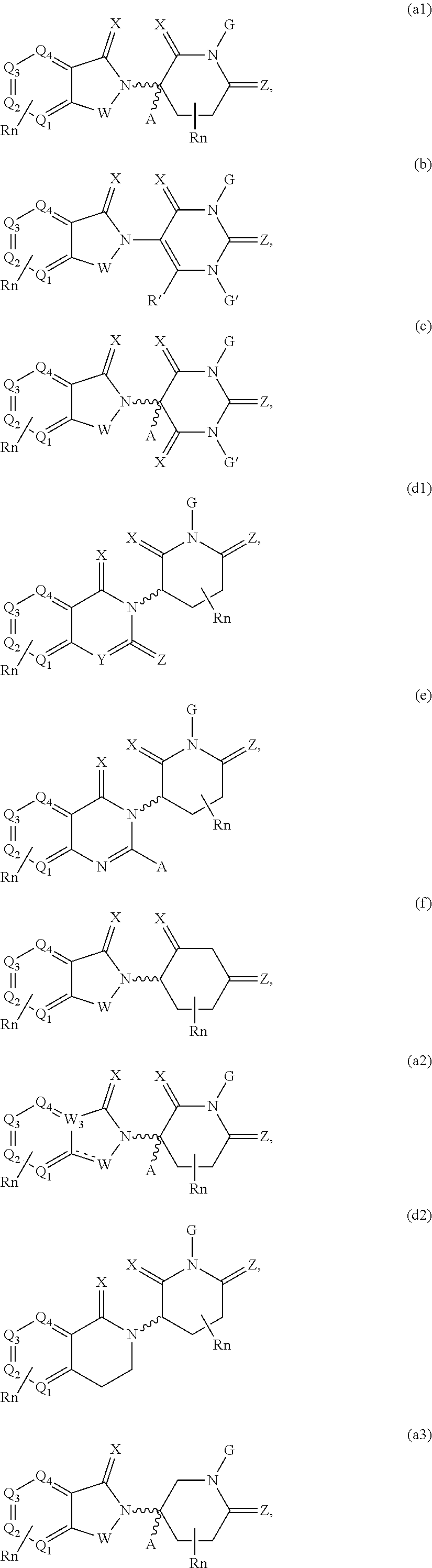
Cereblon ligands and bifunctional compounds comprising the same
a technology of ligands and e3 ligases, applied in the field ofimide-based compounds, can solve the problems of inability to target and modulate certain classes of proteins altogether, difficult to develop ligands of e3 ligases using small molecules, and notoriously difficult to target protein-protein interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[1314]A. Protein Degradation Bioassays:
[1315]The following bioassays evaluate the level of protein degradation observed in various cell types using representative compounds disclosed herein.
[1316]In each bioassay, cells were treated with varying amounts of compounds encompassed by the present disclosure. The degradation of the following proteins may be evaluated: estrogen receptor α (ERα), bromodomain-containing protein 4 (BRD4), androgen receptor (AR), and BRaf protein.
[1317]1. ERE Luciferase Assay for Compounds in Table 5.
[1318]T47D-KBlue cells (ATCC® #CRL_2865, T47D human breast cancer cells stably transfected with estrogen responsive element / promoter / luciferase reporter gene) were seeded into 96-well white opaque plates in RPMI growth medium supplemented with 10% fetal bovine serum (FBS) and allowed to adhere overnight in a 37° C. humidified incubator. The following day, cells were treated with PROTACs in a 12-point concentration curve (top final concentration of 300 nM with sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Aggregation | aaaaa | aaaaa |
| Degradation properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com