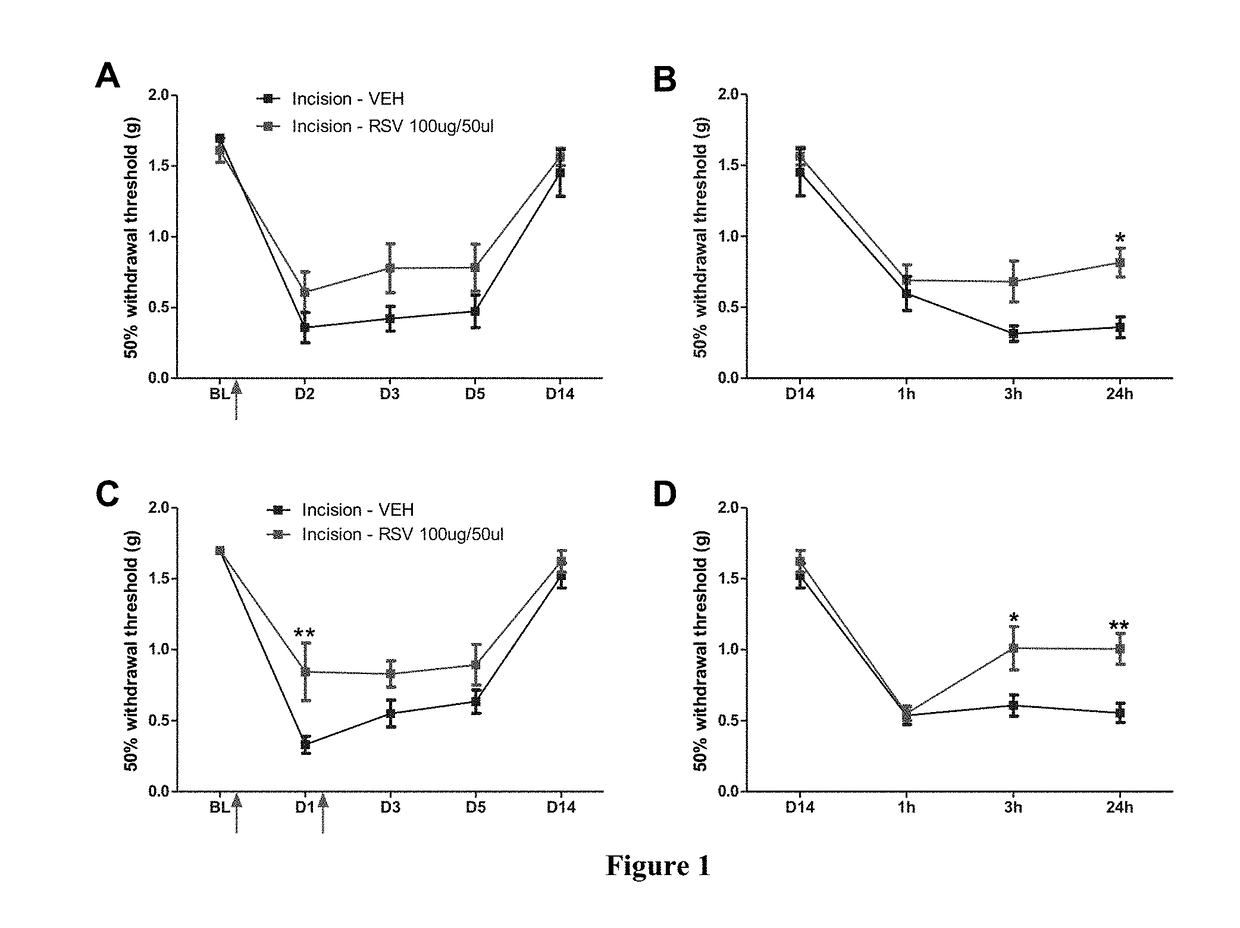
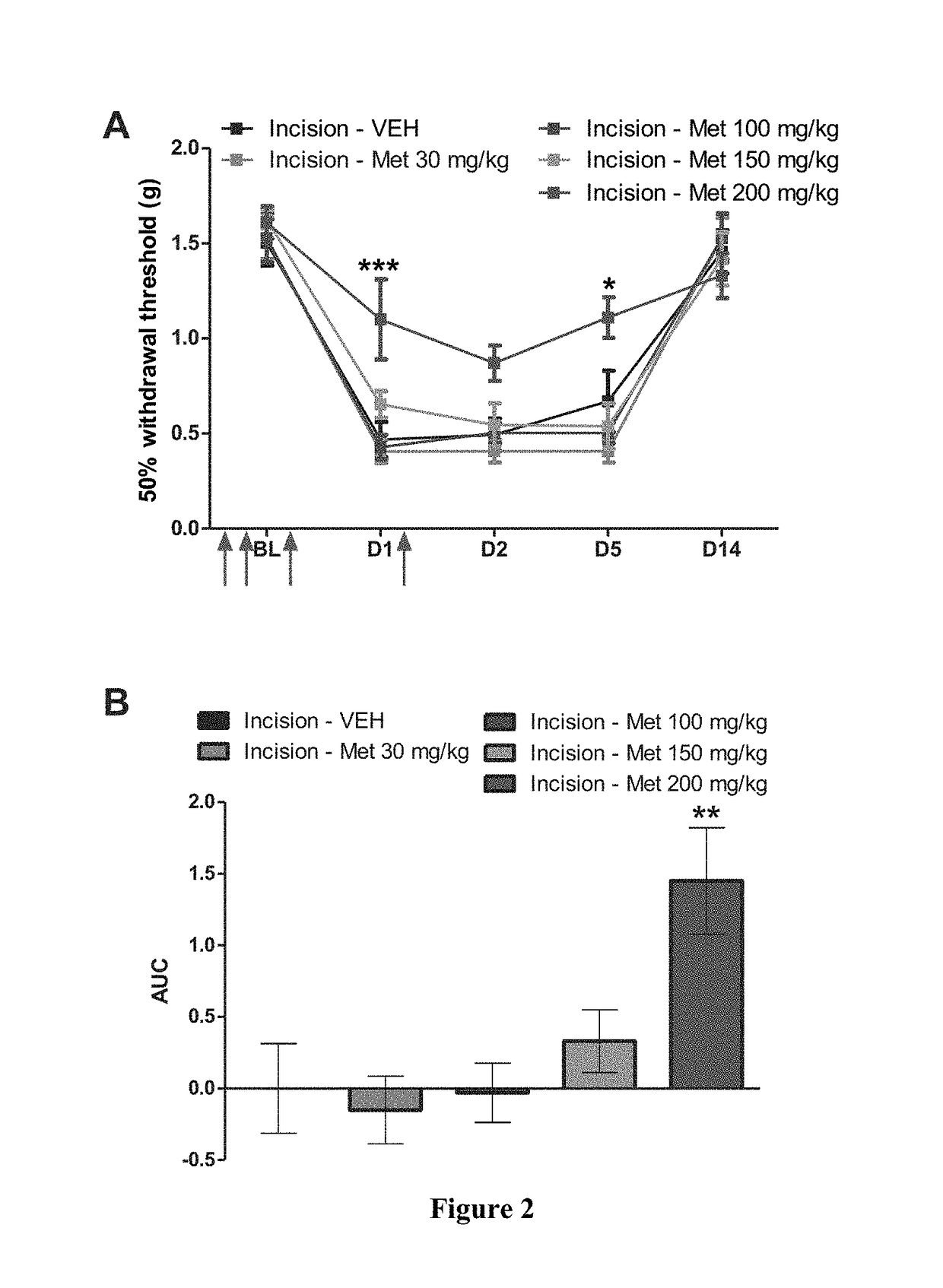
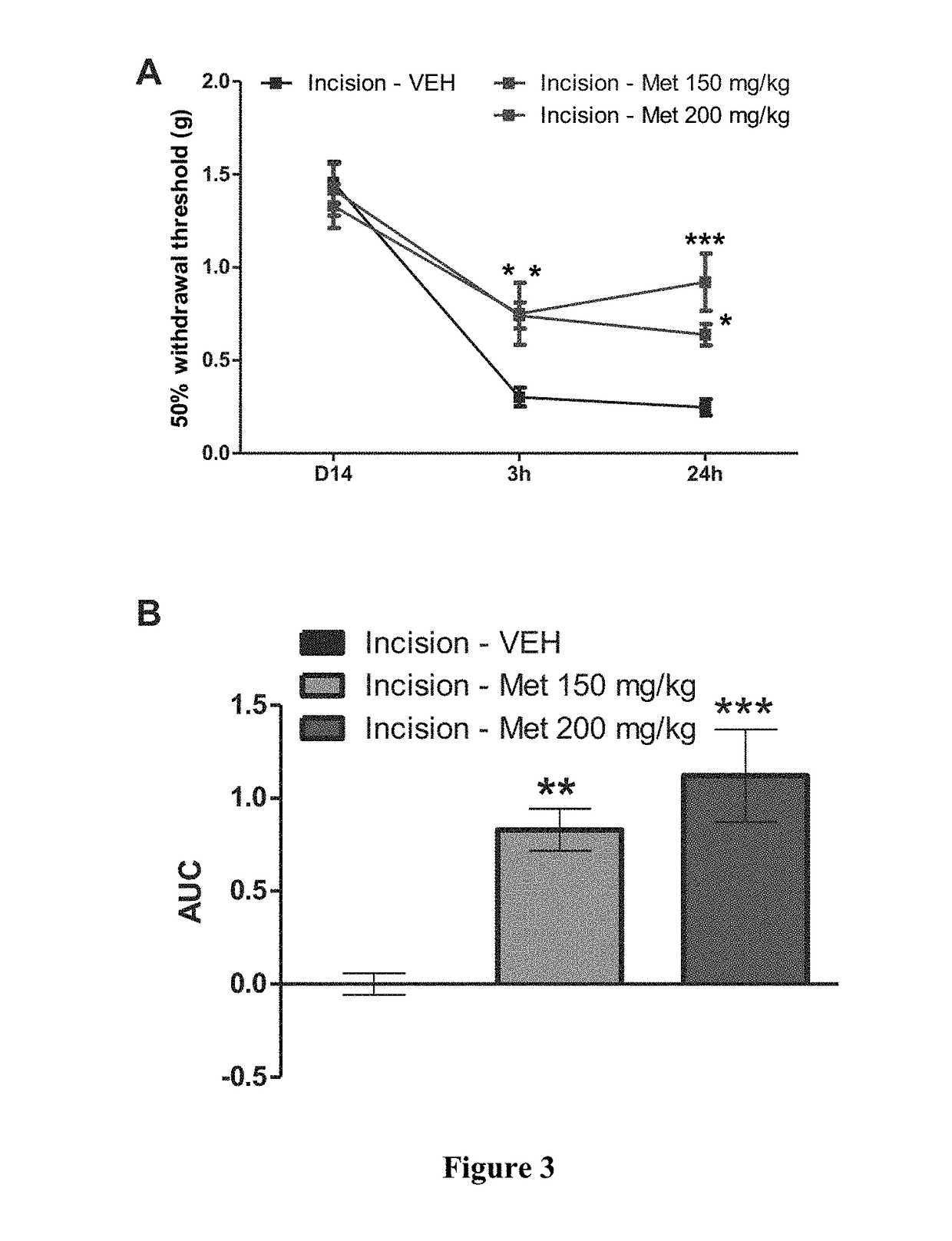
Methods and compositions using ampk activators for pharmacological prevention of chronic pain
a technology of ampk activator and composition, applied in the field of pain treatment, can solve the problems of nsaids) not always preventing the transition to chronic post-surgical pain, and many patients suffer adverse gastrointestinal discomfort, so as to reduce or prevent the development of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0071]Materials and Methods
[0072]Experimental Animals:
[0073]Male ICR mice (Harlan, 20-25 g) were used for the study. All animal procedures were approved by the Institutional Animal Care and Use Committee of The University of Arizona and were in accordance with International Association for the Study of Pain guidelines.
[0074]Behavior Testing:
[0075]For the testing, animals were placed in acrylic boxes with wire mesh floors and allowed to habituate for approximately 1 h on all testing days. Paw withdrawal thresholds were measured using calibrated von Frey filaments (Stoelting, Wood Dale, Ill.) by stimulating the plantar aspect of left hind paw using the up-down method (Chaplan et al., 1994).
[0076]Plantar Incision and Behavioral Testing:
[0077]Prior to surgery all animals were assessed for paw withdrawal thresholds. A mouse model of incisional pain was used for this study (Banik et al., 2006). A 5 mm longitudinal incision was made with a number 11 blade through skin, fascia and muscle of...
example 2
[0096]Case Study
[0097](1) Patient A, a 28 year old female, presents for a scheduled cesarean section. Vial signs and labs appear normal. Obstetrician makes a low transverse incision, and delivery proceeds normally. Approximately 18 hours after surgery, Patient A is administered a sub-efficacious dose of biguanide AMPK activator metformin and a sub-efficacious dose of AMPK activator A769662 to help prevent incision-induced pain such as chronic pain development. Follow up with Patient A regarding incision-induced pain occurs bi-weekly for six weeks post-op. Patient A experiences little incision-induced pain within 5 days post-op, and no pain is observed by 3 weeks post-op.
[0098](2) Patient B, a 55 year-old male, present to surgery for gastric banding surgery. Before anesthesia is initiated, Patient B is administered a sub-efficacious dose of biguanide AMPK activator phenformin and a sub-efficacious dose of AMPK activator leptin to help prevent incision-induced pain such as chronic pai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com