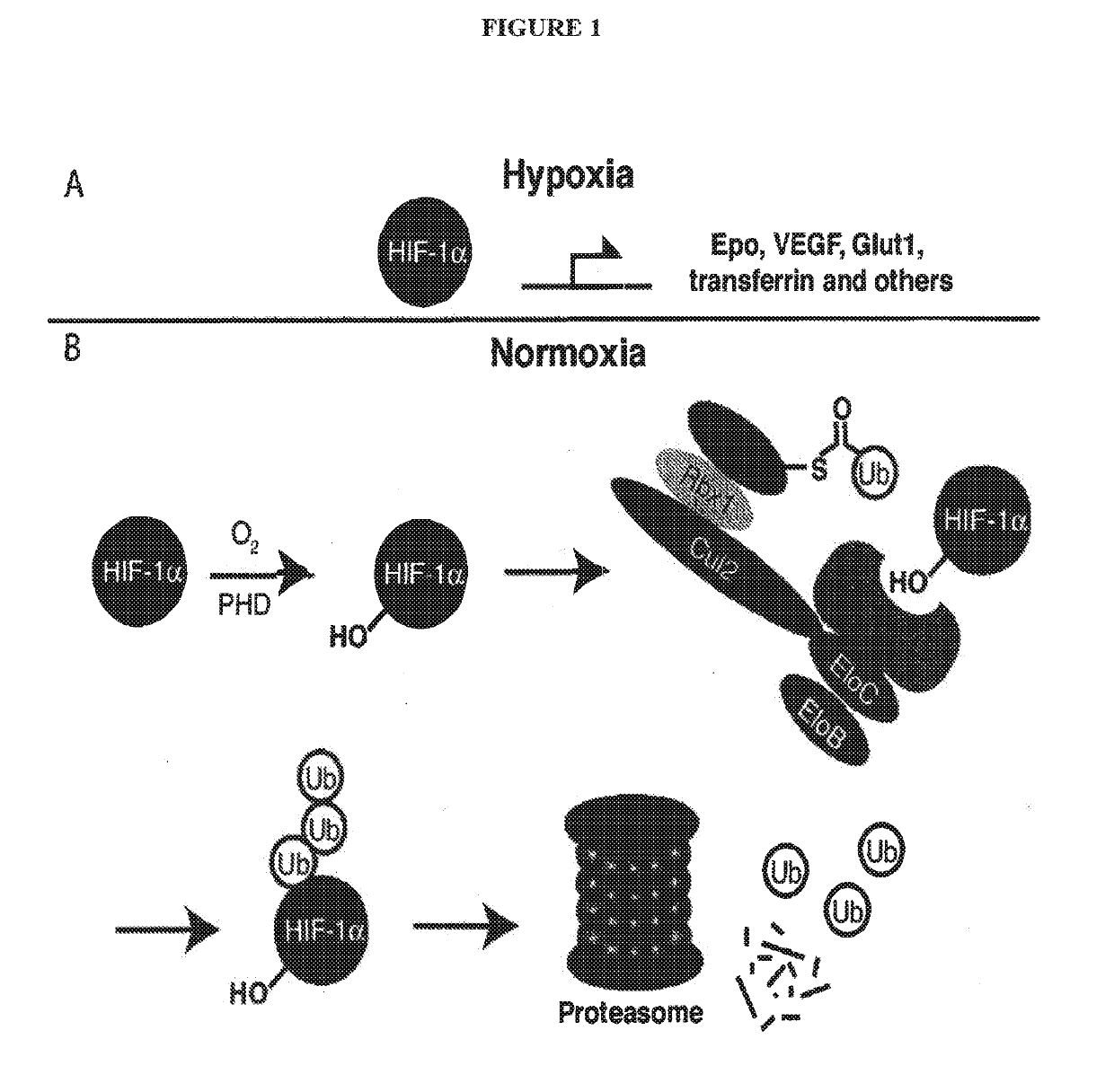
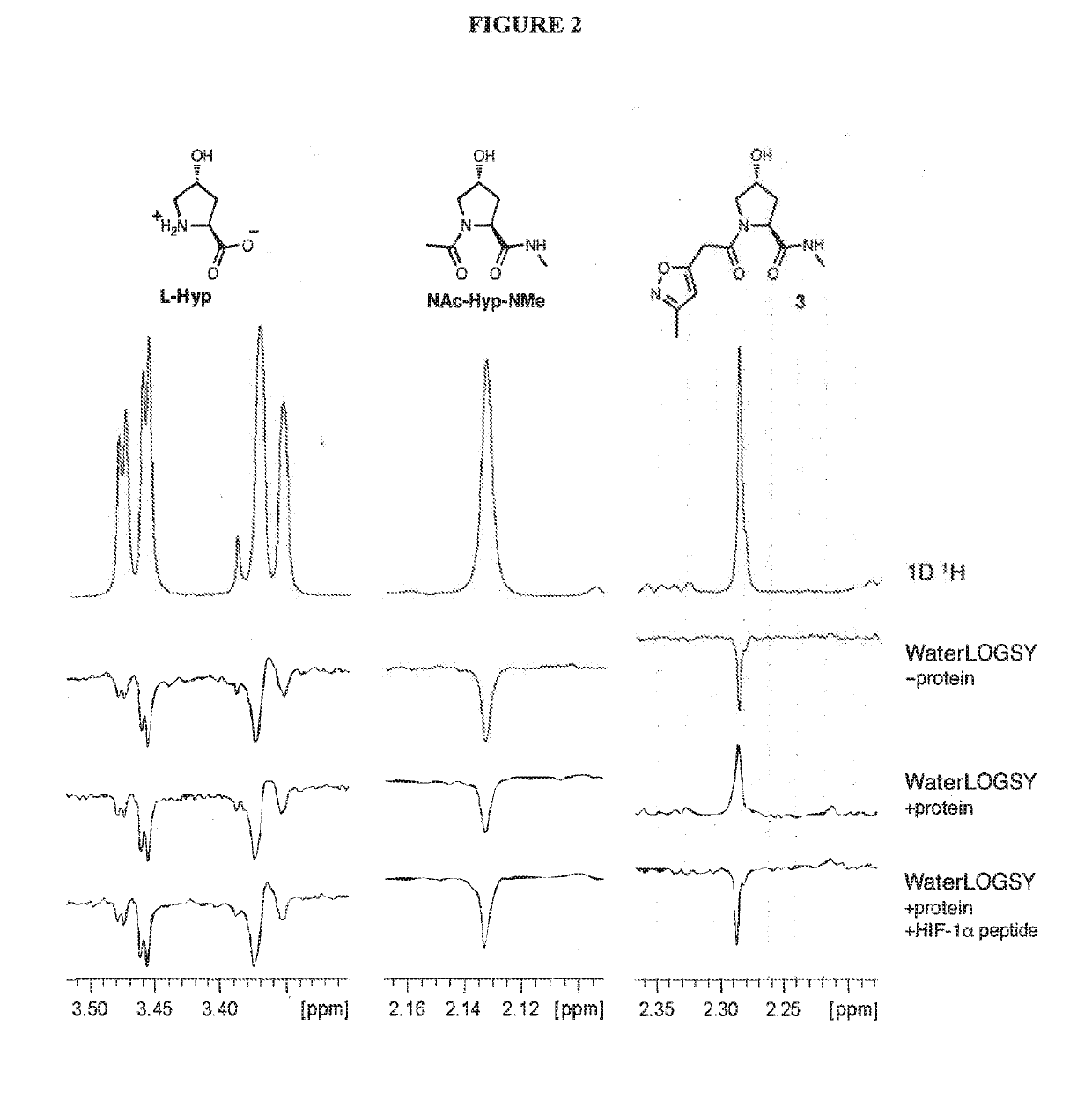
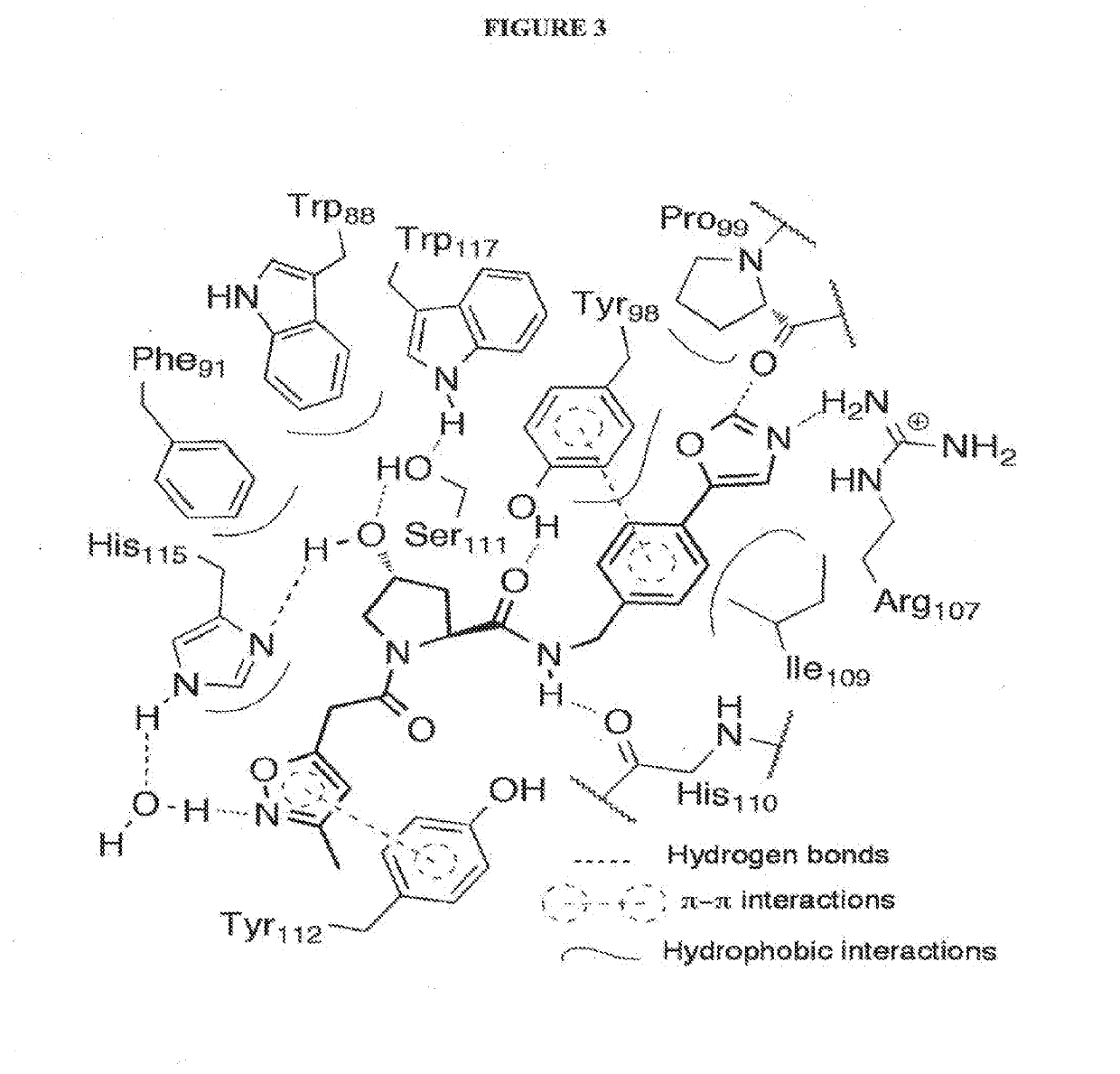
Compounds & Methods for the Enhanced Degradation of Targeted Proteins & Other Polypeptides by an E3 Ubiquitin Ligase
a technology of ubiquitin ligase and compound, which is applied in the field of bifunctional compounds, can solve the problems of notoriously difficult to target the interaction between proteins and proteins using small molecules, and achieve the effect of improving the efficiency of the target protein and the target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples second
Set
[0307]
[0308]VL50 was synthesized from Fmoc-Hyp(OWang)-OAllyl resin (0.156 mmol) according to Solid Phase Synthesis General Method B. It was isolated as a white solid (29.8 mg, 0.084 mmol, 54%). 1H NMR (400 MHz, CD3OD): δ7.34-7.27 (m, 4H); 5.43-5.35 (m, 4H); 3.81-3.78 (dd, J=8 Hz, 4 Hz, 1H); 3.61-3.57 (m, 1H); 2.65-2.61 (m, 2H); 2.57-2.51 (m, 2H); 2.28-2.21 (m, 1H); 2.08-2.02 (m, 1H). 13C NMR (100 MHz, CD3OD): δ 177.53, 174.74, 173.76, 138.75, 133.76, 129.96, 129.49, 70.71, 60.55, 56.47, 43.25, 39.33, 30.97, 30.64. MS (ESI) 354.2 (M+H).
[0309]VL52 was synthesized from Fmoc-Hyp(OWang)-OAllyl resin (0.156 mmol) according to the Solid Phase Synthesis General Method B. It was isolated as a white solid (7.7 mg, 0.021 mmol, 14%). 1H NMR (500 MHz, CD3OD): δ7.41 (d, J=2 Hz, 1H); 7.30 (s, 4H); 6.35-6.34 (dd, J=3 Hz, 2 Hz, 1H); 6.26-6.25 (d, J=3 Hz, 1H); 4.49-4.32 (m, 4H); 3.82-3.73 (m, 3H); 3.65-2.62 (m, 1H); 2.23-2.22 (m, 1H); 2.09-2.06 (m, 1H).13C NMR (125 MHz, CD3OD): δ174.54, 170.58, 14...
examples
(2S,4R)-1-(2-ethoxybenzoyl)-4-hydroxy-N-(4-(oxazol-5-yl)benzyl)pyrrolidine-2-carboxamide
[0423]
[0424]A solution of 2-ethoxybenzoic acid (commercially available from for example Aldrich) (29 mg, 0.17 mmol), (2S,4R)-4-hydroxy-N-(4-(oxazol-5-yl)benzyl)pyrrolidine-2-carboxamide (50 mg, 0.17 mmol) and DIPEA (0.061 mL, 0.35 mmol) in DMF (1 mL) was treated with HATU (80 mg, 0.21 mmol) and the mixture was stirred at ambient temperature for 2 hours. The product was then subjected to purification by mass-directed automated preparative HPLC (formic acid modifier) to afford the title compound (38 mg, 0.087 mmol, 50% yield). LCMS RT=0.72 min, ES+ve m / z 436 [M+H]+.
[0425]Using a method analogous to that for (2S,4R)-1-(2-ethoxybenzoyl)-4-hydroxy-N-(4-(oxazol-5-yl)benzyl)pyrrolidine-2-carboxamide, the following compounds were prepared:
NameStructureYieldRT[M + H]+(2S,4R)-1-benzoyl-4-hydroxy- N-(4-(oxazol-5- yl)benzyl)pyrrolidine-2- carboxamide82%0.65 min392(2S,4R)-4-hydroxy-1-(3- methoxybenzoyl)-N-(4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transparency | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com