Control unit assembly
a control unit and control unit technology, applied in the field of pneumatic medical devices, can solve the problems of inconvenient use, tissue breakdown, and reduce the residence time of blood supplied to the lower limb, so as to improve patient compliance, reduce the number of valves needed in the control unit to control the plurality of inflatable cells, and improve the quality of patient life and control unit li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
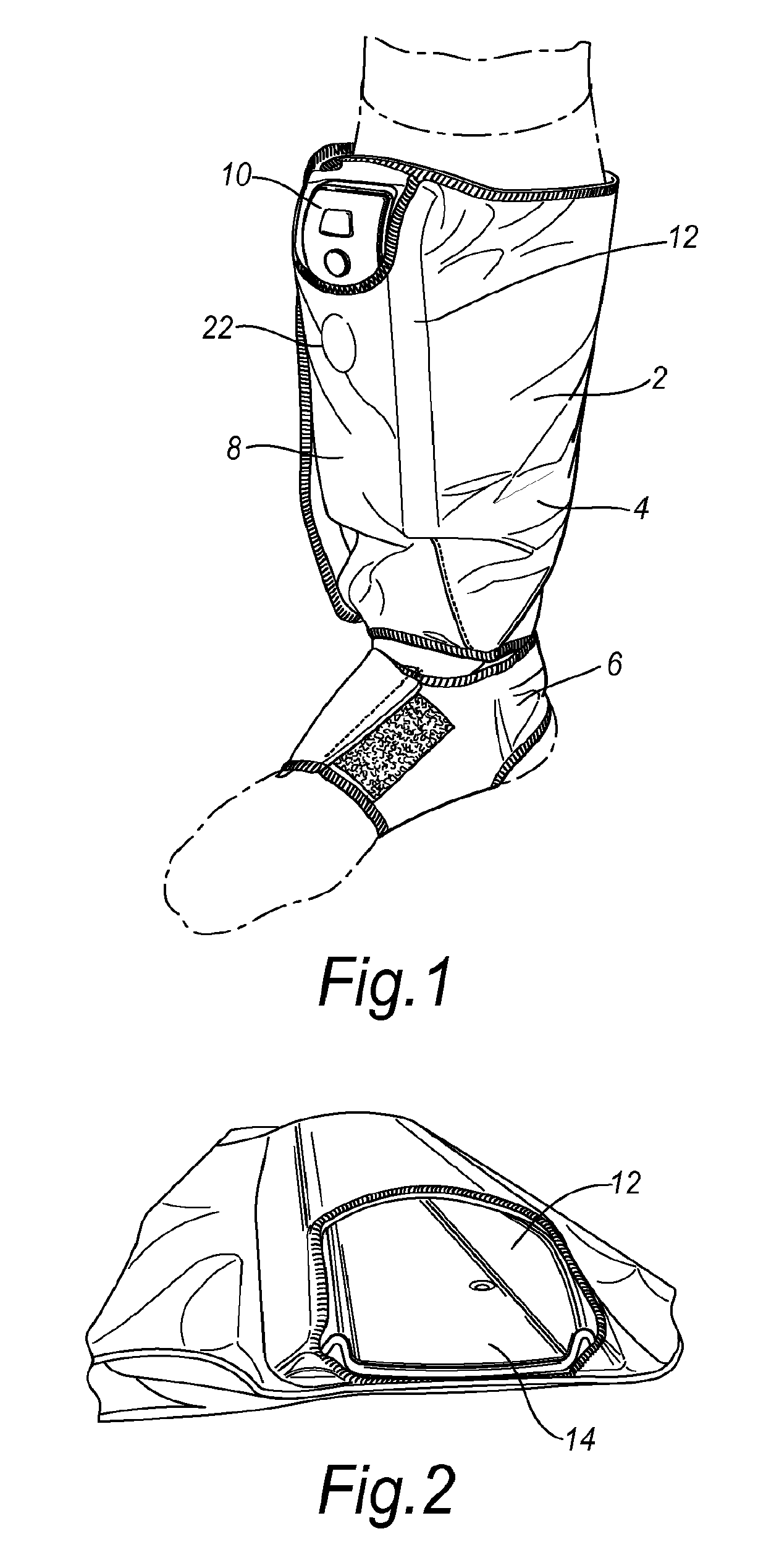
[0042]In FIG. 1 a control unit assembly and compression device according to an embodiment of the invention is shown worn on the leg of a patient. The device comprises a sleeve 2 having a leg cuff 4 connected to a foot cuff 6. The device also comprises a control unit assembly 8 comprising a control unit 10. The control unit 10 is small and when removed from the sleeve 2 may be hand held. The control unit 10 is battery powered and rechargeable so that it can be recharged when attached to or detached from the sleeve 2. FIG. 1 also shows the pouch 12 provided on sleeve 2 for receiving the control unit 10 and the low profile of the assembly. The control unit assembly follows the contour of the device and integrates the assembly into the device.
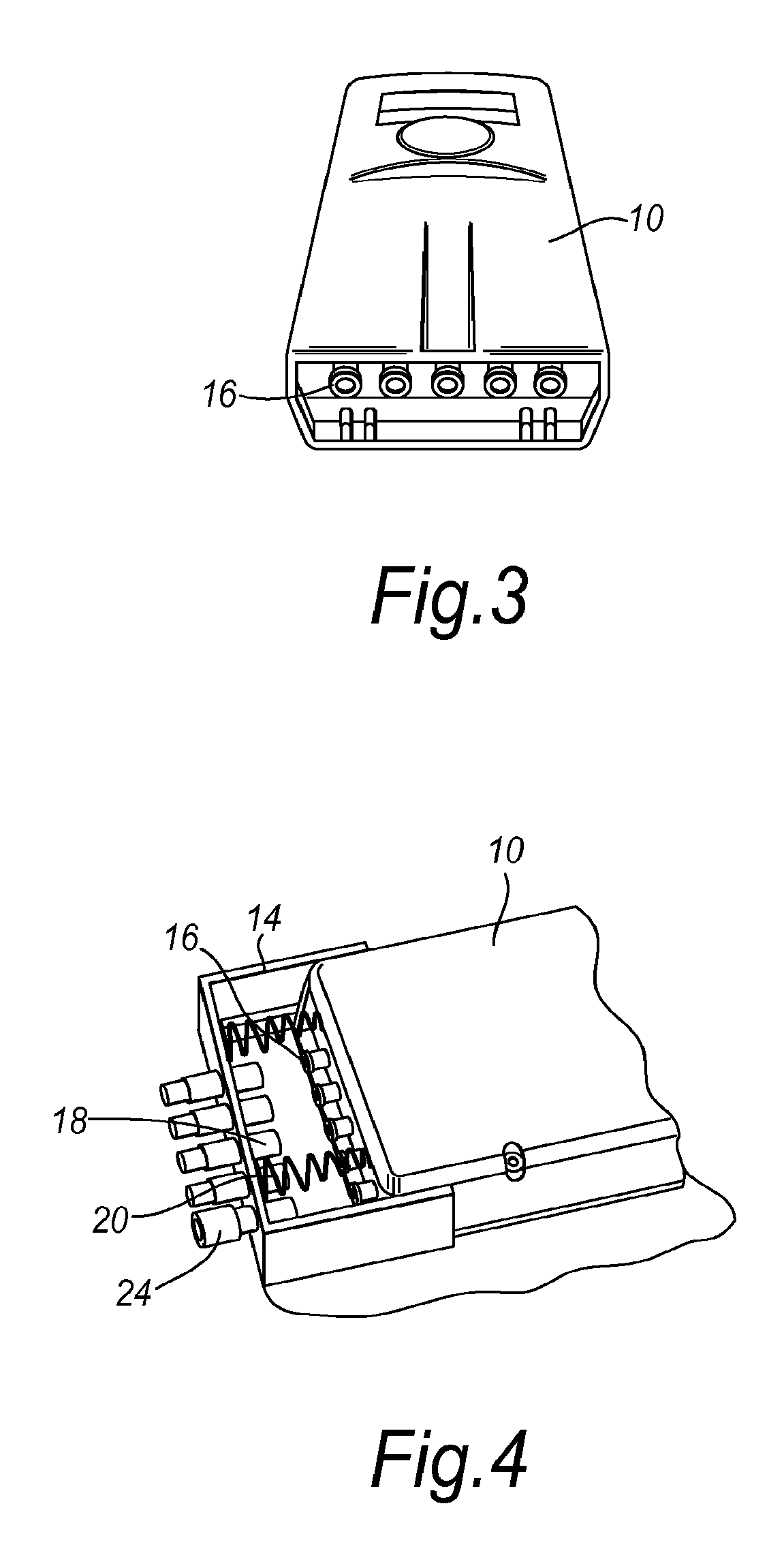
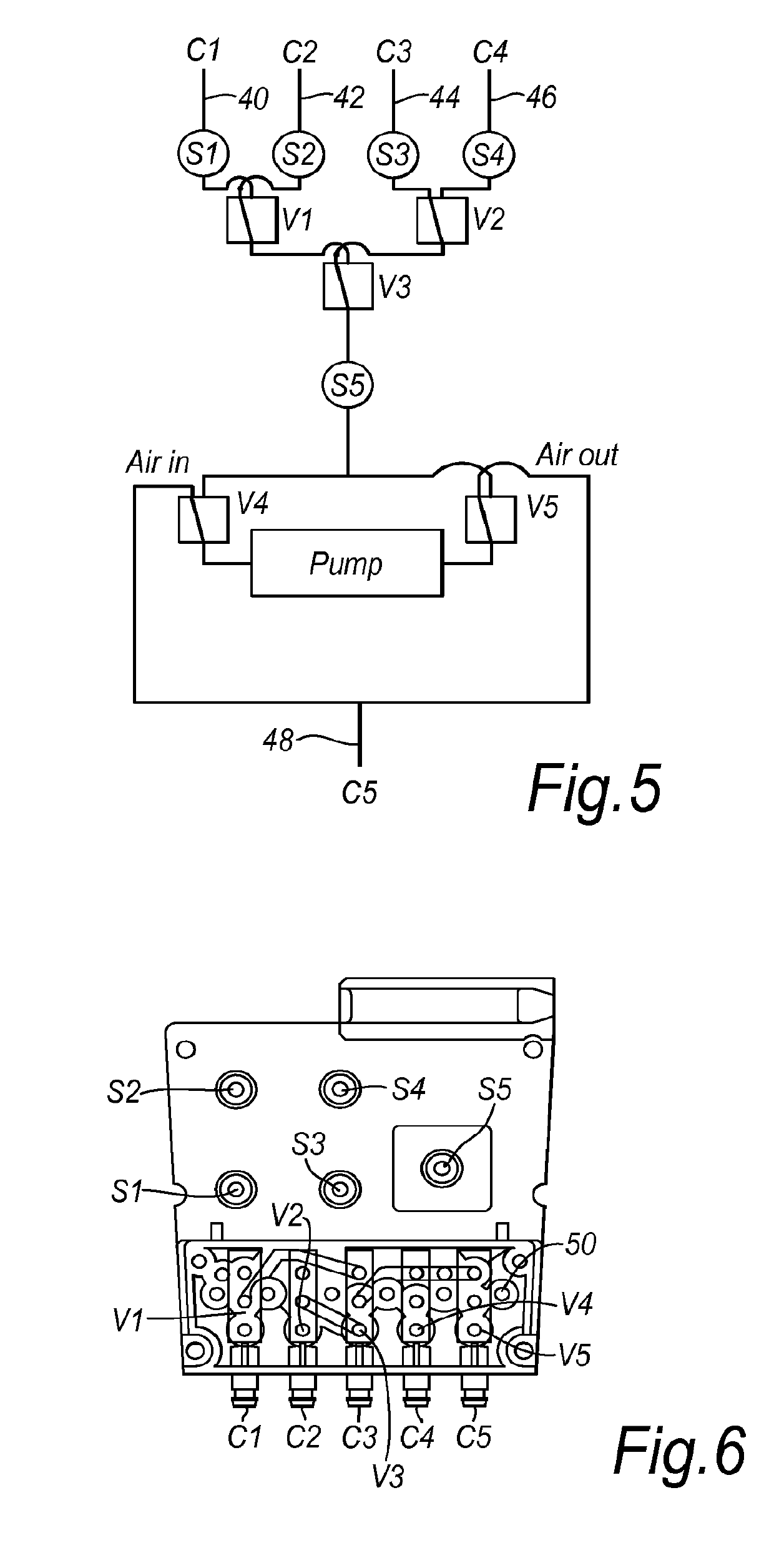
[0043]FIG. 2 is a perspective view taken from above the device with the control unit 10 removed showing the interior of the pouch 12 and the backing plate 14. The control unit 10 may be slidably engaged in the pouch 12 and retained in position by a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com