Treatment of demyelinating diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ne Promotes Functional Recovery from Paralysis when Administered Therapeutically in the Experimental Autoimmune Encephalomyelitis (EAE) Model of MS
[0299]Experimental Detail:
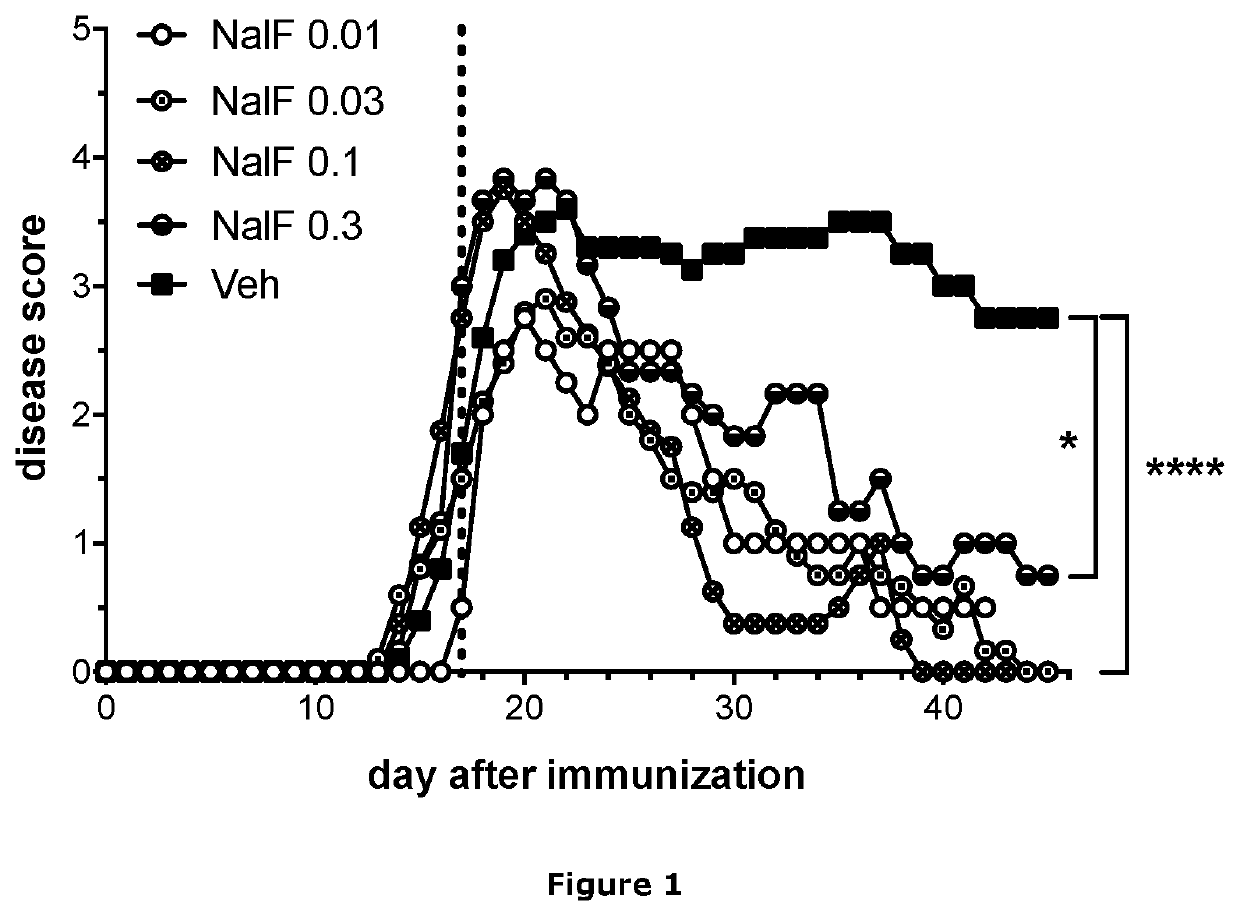
[0300]Female, C57BL / 6 mice were immunized subcutaneously (s.c.) in the hind flanks to induce EAE using myelin oligodendrocyte glycoprotein (MOG) peptide 35-55 (50 mg / mouse) in complete Freund's adjuvant containing heat-killed Mycobacterium tuberculosis (500 μg / mouse). In addition, pertussis toxin (200 ng / mouse) was administered intraperitoneally (i.p.) on days 0 and 2. Mice were weighed and scored daily. On day 17 (vertical dotted line in FIG. 1), mice were started on daily treatment with vehicle only (Veh; 10% tween and 10% DMSO in saline) or nalfurafine at 0.3, 0.1, 0.03, or 0.01 mg / kg by i.p. injection. Nalfurafine was obtained from the University of Kansas, Synthetic Chemical Biology Core Laboratory (97.6% pure by HPLC). Treatment allocation was blinded. The disease was scored from 0-5 with 0 (normal), 1 (par...
example 2
ne Reduces Total Disability when Administered Therapeutically in the EAE Model of MS
Experimental Detail:
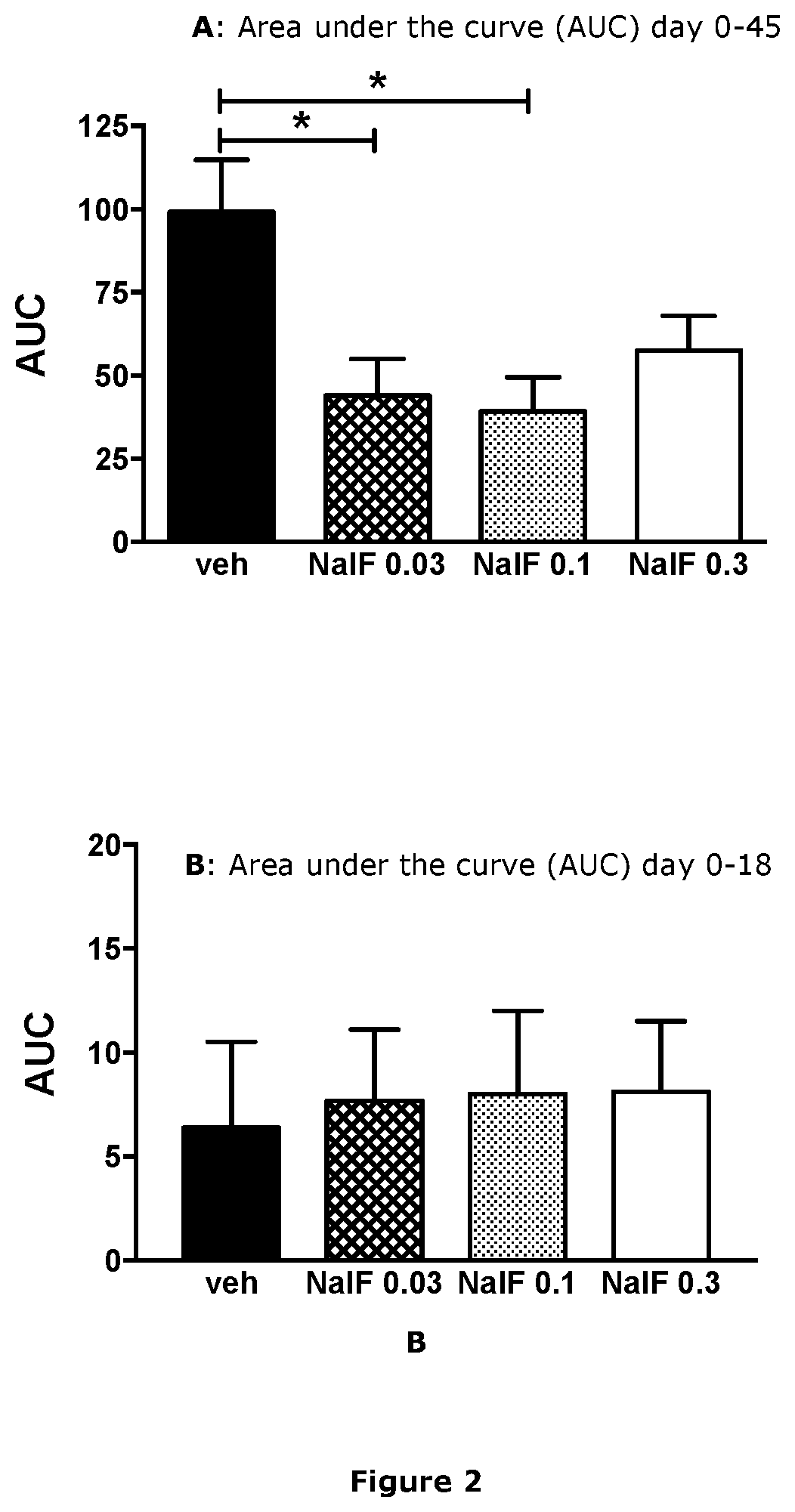
[0302]EAE was induced in female C57BL / 6 mice as described in Example 1. On day 17, mice were started on daily treatment with vehicle only (Veh) or nalfurafine at 0.3, 0.1, or 0.03 mg / kg by i.p. injection. The area under the curve (AUC) was calculated for each mouse based upon the daily disease score and represents the total disability experienced. Shown in FIG. 2 are results from 1 representative experiment. * p<0.05 by one-way ANOVA with Dunnett's multiple comparison test.
Interpretation and Impact:
[0303]Despite all treatment groups having similar disease scores at the start of treatment (lower graph), mice treated daily with nalfurafine had significantly lower total disability by day 45 after immunization to induce EAE (upper graph). Doses of 0.03 and 0.1 mg / kg nalfurafine had the greatest effect at reducing disability. The 0.1 mg / kg nalfurafine dose results in a 60% reduction in...
example 3
ne Promotes Recovery from EAE-Induced Weight Loss when Administered Therapeutically
Experimental Detail:
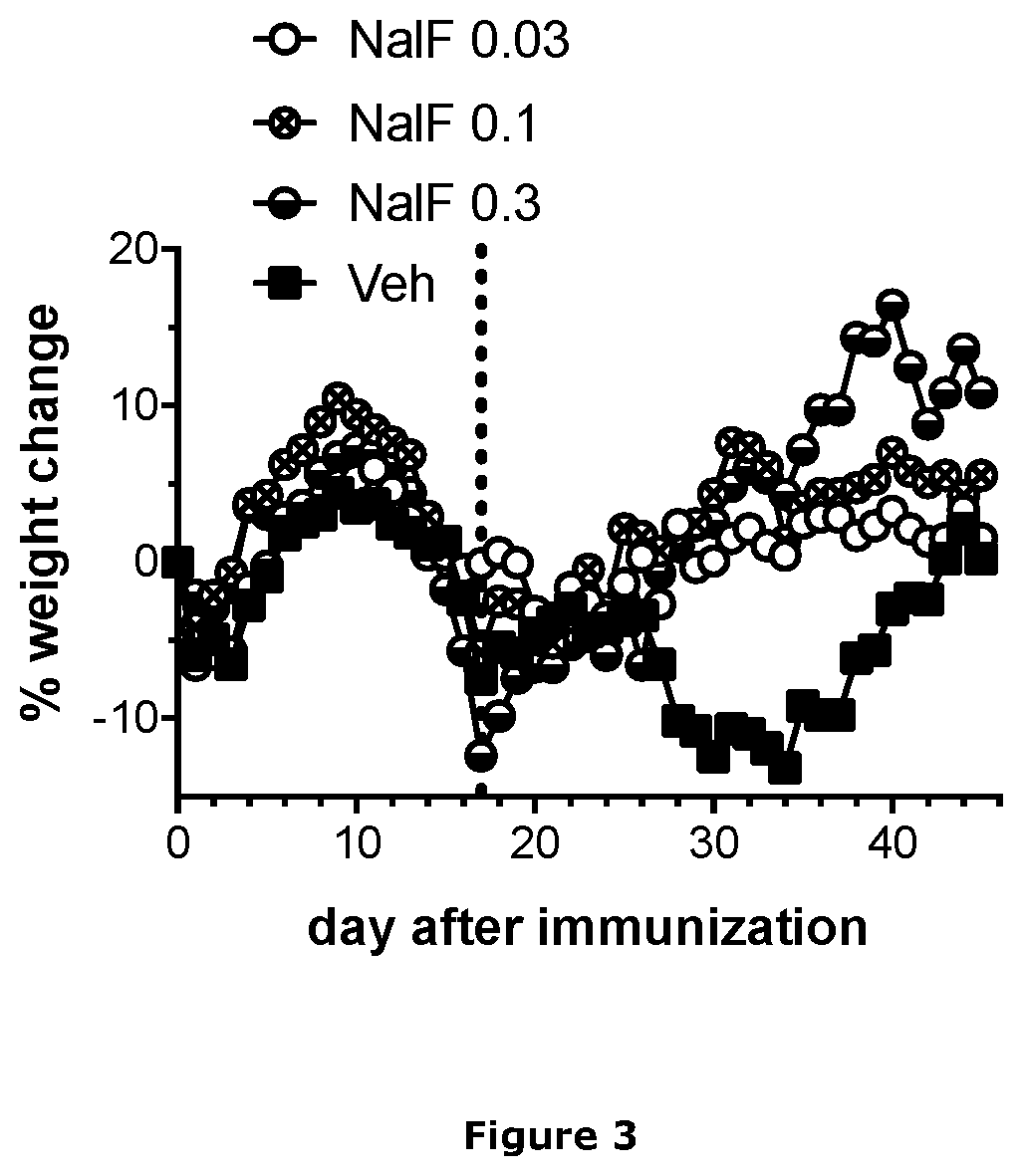
[0305]EAE was induced in female C57BL / 6 mice as described in Example 1. Mice were weighed daily and the % change in body weight calculated. On day 17 (vertical dotted line in FIG. 3), mice were started on daily treatment with vehicle only (Veh) or nalfurafine at 0.3, 0.1, or 0.03 mg / kg by i.p. injection.
Interpretation and Impact:
[0306]As shown in FIG. 3, at onset of disease, mice rapidly lose weight. Once treatment with nalfurafine is initiated (vertical dotted line), mice recover from EAE-induced weight loss.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com