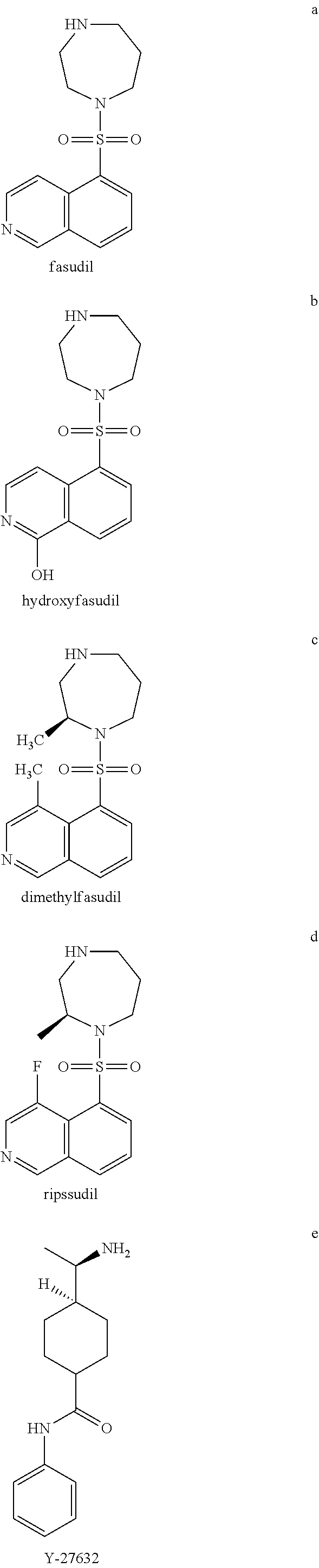
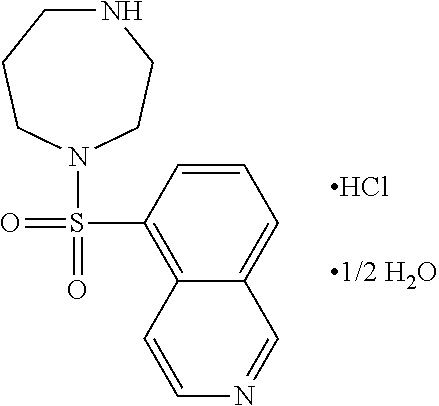
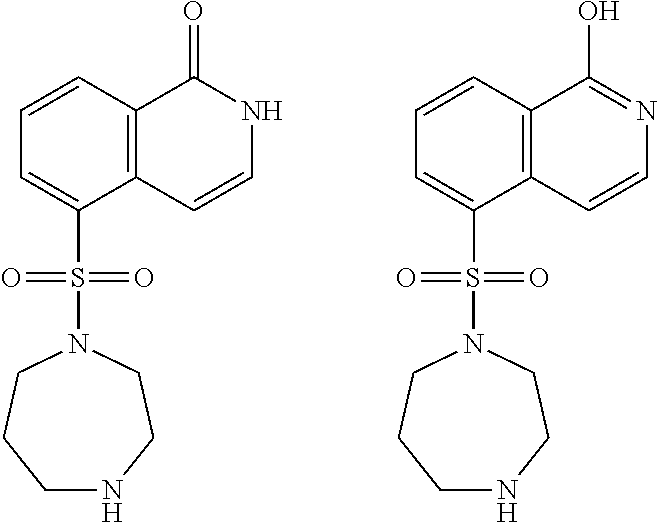
Methods of treating 4-repeat tauopathies
a tauopathy and repeating technology, applied in the field of repeating tauopathy, can solve the problems of not being able to effectively treat any 4r tauopathy, pharmacological approaches and especially those aimed at disrupting tau pathology do not work, and the approach to disrupting tau pathology will not work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0092]Ten subjects 50-85 years old, meeting the National Institute of Neurological Disorders and Stroke-Society for Progressive Supranuclear Palsy (NINDS-SPSP) probable or possible PSP criteria, (Litvan 1996) as modified from the AL-108-231 trial (Boxer 2014) are enrolled. MRI at screening is consistent with PSP (≤4 microhemorrhages and no large strokes or severe white matter disease). They have Mini-Mental State Examination (MMSE) score 14-30 and have stable medications for 2 months prior to screening, including FDA approved Alzheimer's disease (AD) medications and Parkinson's disease medications. Subjects are excluded if they meet the National Institute on Aging-Alzheimer's Association Workgroups criteria for probable AD (McKhann 2011), if they have any medical condition other than PSP that could account for cognitive deficits (e.g., active seizure disorder, stroke, vascular dementia) or if they have a prominent and sustained response to levodopa therapy (suggestive of Parkinson's...
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com