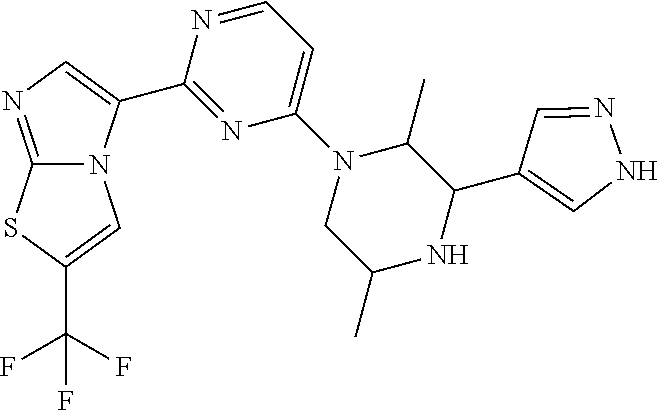
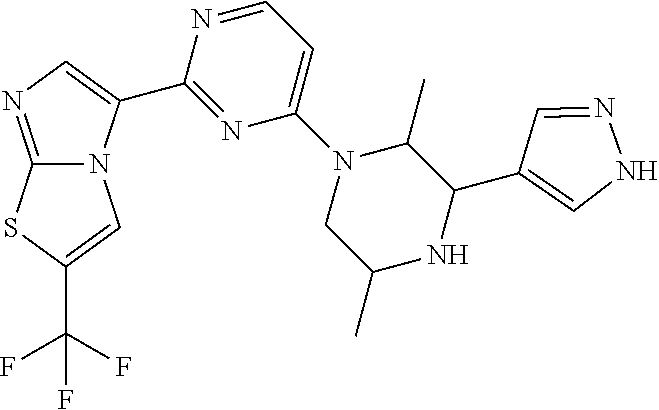
Gcn2 inhibitors and uses thereof
a technology of gcn2 and inhibitors, applied in the field of gcn2 inhibitors, can solve the problems of reducing the translation of most and reducing the global utilization of amino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
S)-1-[6-[6-(Difluoromethyl)imidazo[1,2-b]pyridazin-3-yl]pyrimidin-4-yl]-4,4-difluoro-5-methyl-3-piperidyl]methyl]methanesulfonamide, II-36
[1456]
[1457]3-(6-Chloropyrimidin-4-yl)-6-(difluoromethyl)imidazo[1,2-b]pyridazine (511.5 mg, 1.82 mmol), N-[[(3S,5S)-4,4-difluoro-5-methyl-3-piperidyl]methyl]methane sulfonamide (400 mg, 1.65 mmol) and DIPEA (426.8 mg, 575.2 μL, 3.30 mmol) were combined in NMP (5 mL) and stirred at 80° C. for 16 hours. The mixture was filtered through a Whatman filter, washing with DMSO (8 mL) and the resulting solution was purified by reverse phase chromatography (C18, MeCN / water / 0.05% TFA as eluent). The clean fractions were then passed through bicarbonate resin cartridges, combined and the resulting solution lyophilised, to give N-[[(3S,5S)-1-[6-[6-(difluoromethyl)imidazo[1,2-b]pyridazin-3-yl]pyrimidin-4-yl]-4,4-difluoro-5-methyl-3-piperidyl]methyl]methanesulfonamide as an off-white solid (428 mg, 53%).
[1458]The following compounds were prepared using a methodo...
example 2
2,4-Triazol-4-yl)methyl)-4-(6-(6-(difluoromethyl)imidazo[1,2-b]pyridazin-3-yl)pyrimidin-4-yl)morpholine, II-29
[1823]
[1824]A mixture of 2-(1,2,4-triazol-4-ylmethyl)morpholine (trifluoroacetate salt) (15 mg, 0.053 mmol), 6-(difluoromethyl)-3-(6-fluoropyrimidin-4-yl)imidazo[1,2-b]pyridazine (14.1 mg, 0.053 mmol) and K2CO3 (40 mg, 0.289 mmol) in NMP (1 mL) was stirred at 90° C. in a sealed tube for 24 hours. The crude mixture was purified by reverse phase chromatography (C18, MeCN / water / 0.05% TFA as eluent). The pure fractions were combined and lyophilised to yield 2-((4H-1,2,4-triazol-4-yl)methyl)-4-(6-(6-(difluoromethyl)imidazo[1,2-b]pyridazin-3-yl)pyrimidin-4-yl)morpholine as a pale yellow solid (10 mg, 45%).
[1825]The following compounds were prepared using a methodology similar to the one described in Example 2:[1826](S)—N-((4-(6-(6-(Difluoromethyl)imidazo[1,2-b]pyridazin-3-yl)pyrimidin-4-yl)-1-methyl-6-oxopiperazin-2-yl)methyl)methanesulfonamide II-7;[1827]N-((1-(6-(6-(Difluorometh...
example 3
(6-(Difluoromethyl)imidazo[1,2-b]pyridazin-3-yl)pyrimidin-4-yl)piperidin-3-yl)methyl)methanesulfonamide, II-59
[1845]
[1846]To 6-(difluoromethyl)-3-iodo-imidazo[1,2-b]pyridazine (65 mg, 0.22 mmol) in dry 1,4-dioxane (4 mL) was added tributyl(tributylstannyl)stannane (255.6 mg, 0.44 mmol), LiCl (46.72 mg, 1.1 mmol) and tetrakis(triphenylphosphine)palladium(0) (12.7 mg, 0.011 mmol). The mixture was heated at 105° C. under nitrogen for 15 hours. N-[[1-(6-Bromopyrimidin-4-yl)-3-piperidyl]methyl]methanesulfonamide (38 mg, 0.11 mmol) and Pd(PPh3)2Cl2 (15.5 mg, 0.022 mmol) were added sequentially and the mixture was stirred at 140° C. in a sealed tube for 4 hours. Another portion of N-[[1-(6-bromopyrimidin-4-yl)-3-piperidyl]methyl]methanesulfonamide (76 mg, 0.22 mmol) was added and the mixture was stirred at 140° C. for a further 4 hours. The mixture was purified by reverse phase chromatography (C18, MeCN / Water 0.05% TFA as eluent) to afford N-((1-(6-(6-(Difluoromethyl)imidazo[1,2-b]pyridazi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com