Method for the treatment of cerebral ischaemia and use of erythropoietin or erythropoietin derivatives for the treatment of cerebral ischaemia
a technology of erythropoietin and cerebral ischaemia, which is applied in the field of method the use of erythropoietin or erythropoietin derivatives for the can solve the problem of no effective treatment of cerebral ischaemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
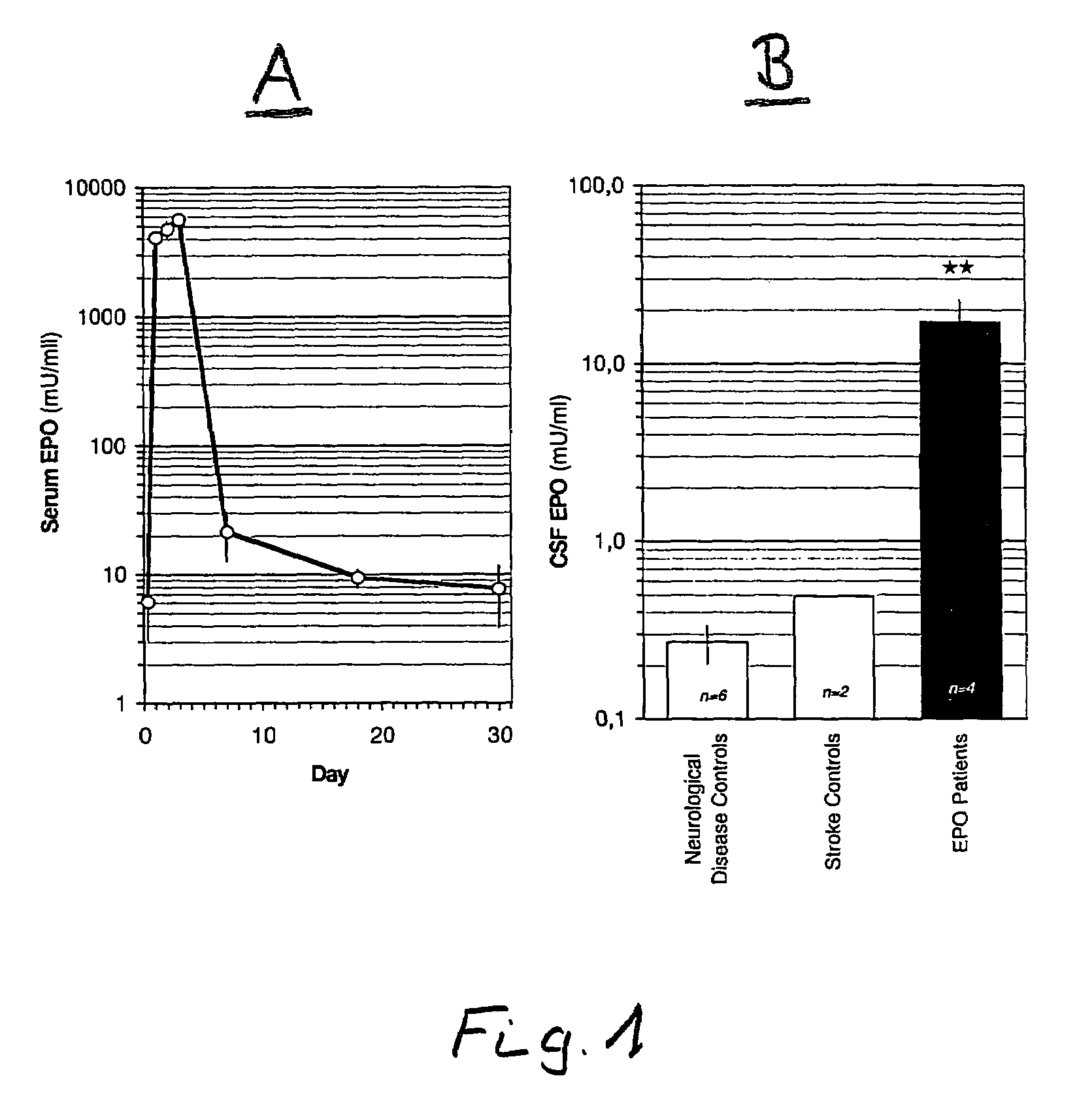
[0026]In FIG. 1A, the average of the serum concentration of four patients with strokes, i.e. whose peripheral concentration of erythropoietin is measured over several days to whom at approximately 8 hours, approximately 24 hours and again approximately 48 hours after the stroke were given respectively a dose of 35,000 IE human recombinant erythropoietin (preparation “Neorecormon” by the Hoffmann LaRoche AG company) intravenously. It can be detected that the serum concentration achieves its maximum within the first few days and then decreases sharply subsequently.
[0027]In FIG. 1B, the concentrations of EPO are represented in six control patients with non-ischaemic neurological illnesses (“neurological disease controls”) after infusion of erythropoietin, in two untreated stroke patients (“stroke controls”) without infusion of erythropoietin and also in four stroke patients (“EPO patients”) after infusion of erythropoietin as in the case of the control patients. There is represented th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com