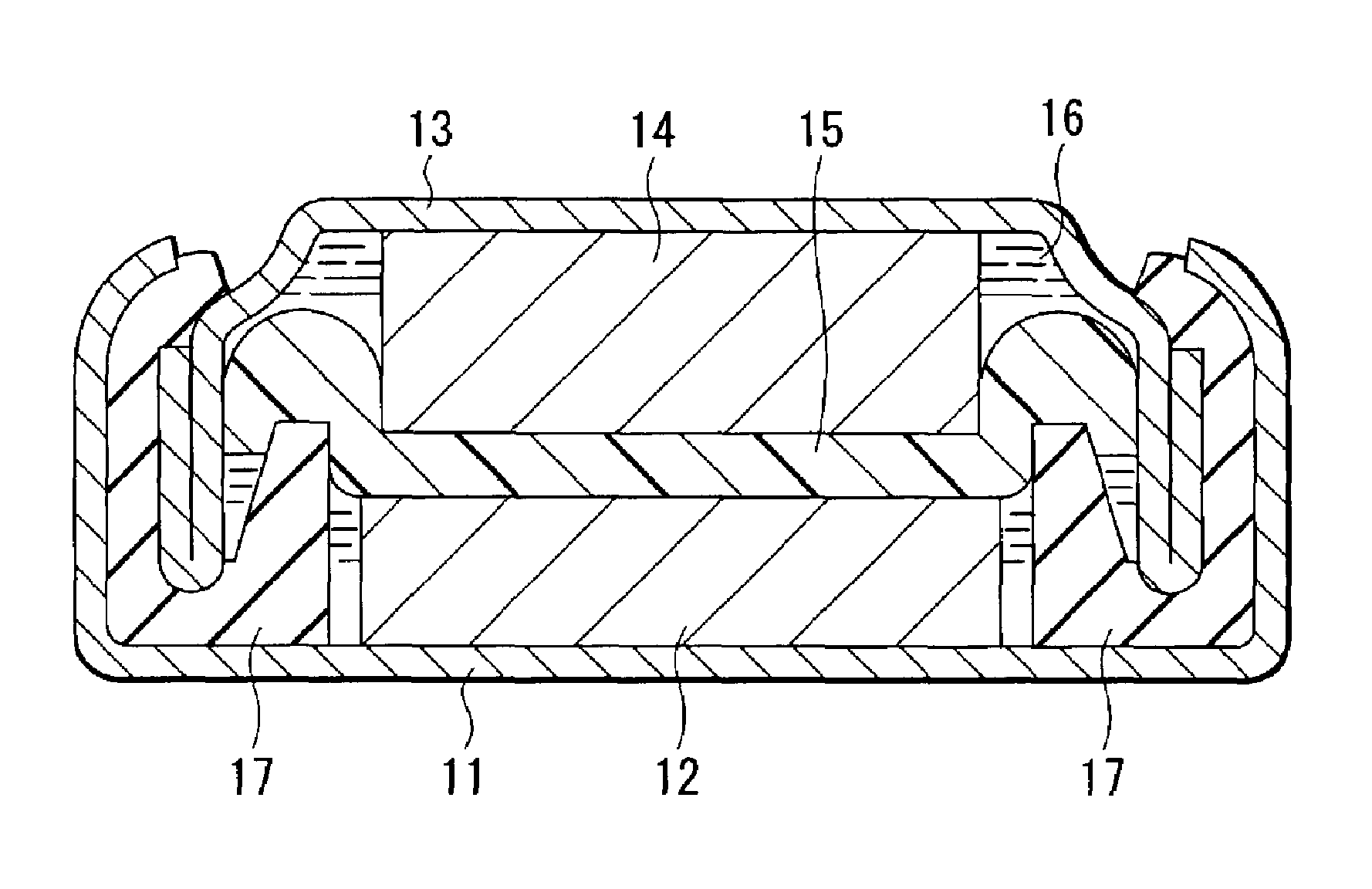
Positive electrode material and battery using the same
a technology of positive electrodes and electrode materials, applied in the direction of positive electrodes, electrochemical generators, cell components, etc., can solve the problems of reducing cost, reducing discharge capacity, and higher material cost, and achieve high discharge potential without reducing capacity, high discharge potential, and high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-5
[0047]First, as shown in Table 1, using the various compounding ratios in Examples 1-5, manganese carbonate (MnCO3), iron oxalate dihydrate (FeC2O4.2H2O), dihydrogen ammonium phosphate (NH4H2PO4), and lithium carbonate (Li2CO3) were mixed, and fully ground with a ball mill. Subsequently, the obtained mixture and acetylene black were mixed at 94:6 of the weight ratio, fully ground and mixed with the ball mill, and then fired at 400° C. −750° C. under nitrogen atmosphere for 24 hours to synthesize compounds of Li1+xMnyFezPO4 having a composition shown in Table 1.
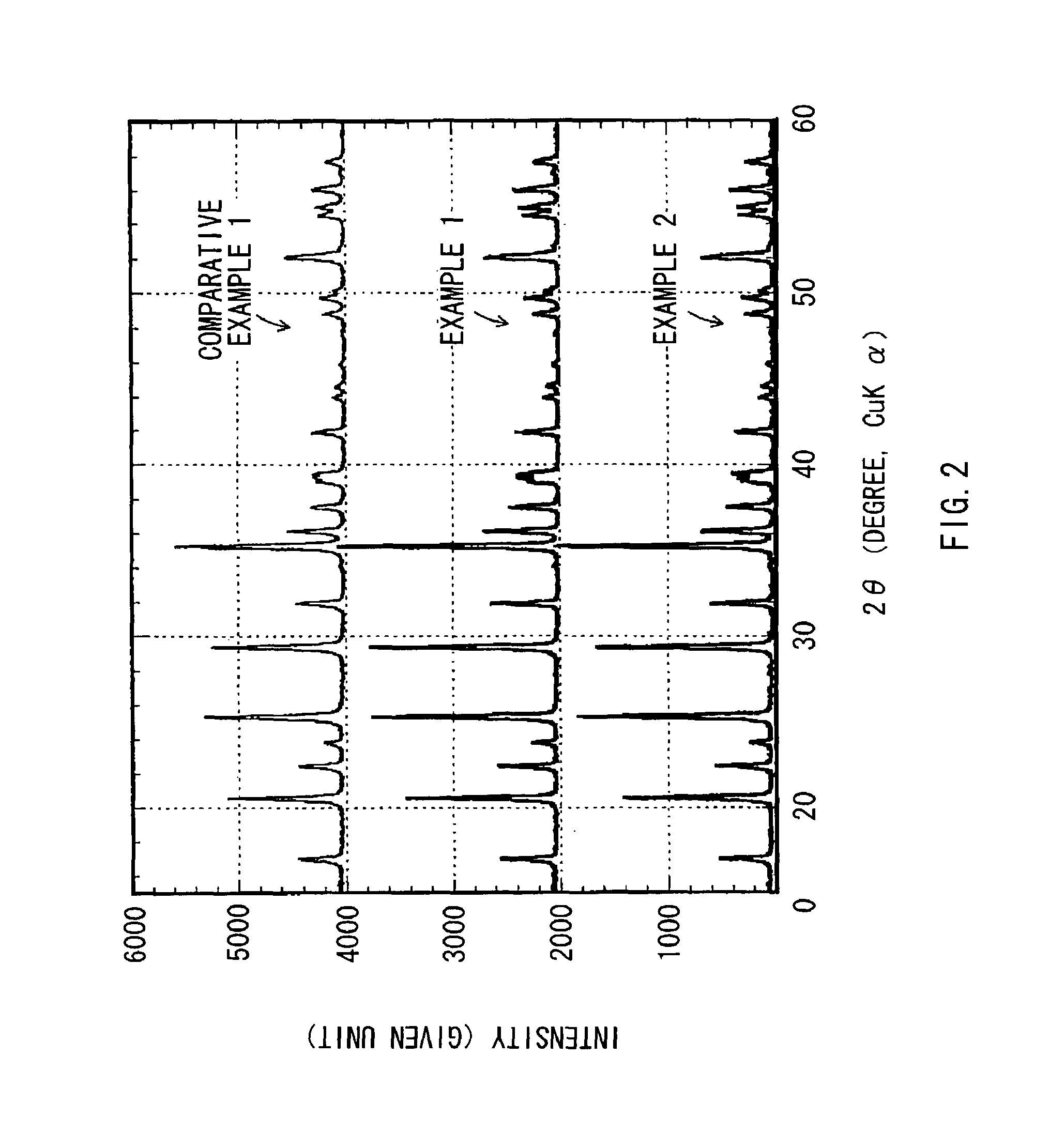
[0048]The X-ray powder diffraction patterns of the obtained compounds of Examples 1-5 were measured. The rotating-anode type of Rigaku RINT2500 was used for an X-ray diffractometer. In this respect, the X-ray diffractometer, equipped with a vertical standard type goniometer of 185 mm goniometer radius, is of the type monochromatizing the x-ray beam with the combination of a pulse-height analyzer and a counter monochromator wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Brunauer-Emmet-Teller specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| volume density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com